In the life sciences industry, Quality Management (QM) has long been synonymous with compliance: checking boxes to meet regulatory demands. But that definition no longer holds up. Today, QM must do much more than ensure compliance; it needs to enable speed, scalability, and sustained innovation across complex, global operations. To do that, prominent gaps need to be filled.
ADVERTISEMENT
It all began with a view from the window of the 15th floor of Bayer’s site in Berlin, reminiscent of Boston before it became one of the most important biotech hotspots in the world. This inspired the vision of developing the Bayer Campus into “Boston an der Spree” – a vibrant healthcare campus that fosters collaboration and innovation.
In life sciences, breakthroughs are reshaping healthcare faster than ever. But true leadership means going beyond the lab – understanding the complex ecosystem where science, policy, and business collide. For those struggling to navigate this complexity, WU Vienna’s Executive MBA offers the insights and connections needed to stay ahead in an unpredictable industry.
The Hamburg-based company Indivumed has repositioned itself, sending a clear message with a recent change in leadership: A new chapter is beginning with the focus on discovering and developing novel therapeutic targets for innovative cancer therapies. |transkript spoke with Prof. Dr. Hartmut Juhl and Dr. Matthias Evers.
UP Catalyst, Far Eastern New Century Corporation, and Oxylus Energy were recognised at the “Best CO2 Utilisation 2025” Innovation Award for transforming carbon emissions into high-performance battery materials, sustainable plastics for footwear and textiles, and methanol fuel, marking major strides toward a renewable carbon future.
The pharmaceutical service provider publishes its 2024 Sustainability Report.
Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) is an industry alliance to support research and development projects in AMR. Since its inception in 2016, CARB-X has supported 114 R&D projects across 14 countries, leading to 18 projects advancing into or completing clinical trials. EBM talked to CARB-X Executive Director Kevin Outterson.
Therapeutic oligonucleotides are rapidly transforming drug development by enabling precise gene modulation strategies. Microsynth empowers biotech, pharmaceutical, and academic researchers with high-quality therapeutic oligonucleotides, supporting every stage from sequence design to preclinical development. With tailored solutions, advanced automation, and expert design support, we accelerate your therapeutic programmes with precision and speed.
The landscape of macromolecule drug discovery is evolving rapidly, with oligonucleotides and peptides leading the way in innovative therapeutic applications. CDD Vault, a leading scientific data management system, significantly improves the way researchers manage macromolecule data by introducing a dedicated Macromolecule mode that seamlessly integrates with modern workflows.
The much-discussed digital transformation in the healthcare sector is in full swing. European Biotechnology spoke with Dr. Sabrina Graf, COO of the globally active Tenthpin Group, about how the right software can improve quality management, keep the system landscape lean, and safe both, time and money.


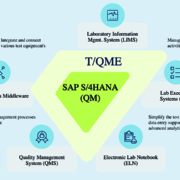 Tenthpin Solutions AG
Tenthpin Solutions AG Bayer AG
Bayer AG WU Vienna University of Economics and Business Administration
WU Vienna University of Economics and Business Administration Indivumed GmbH
Indivumed GmbH

 CARB-X
CARB-X Microsynth AG
Microsynth AG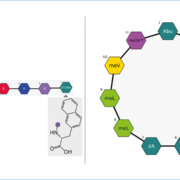 Collaborate Drug Discovery Inc.
Collaborate Drug Discovery Inc. Tenthpin Group
Tenthpin Group