In an interview with European Biotechnology Magazine during J.P. Morgan Week in San Francisco, Dr. Jian Zhang, Acting CEO of BioDlink, explains how a partnership-first approach and deep ADC expertise help him offer biotech companies a competitive edge.
ADVERTISEMENT
Tag Archive for: Drug development
UK-based biotech LoQus23 Therapeutics is advancing an oral small molecule targeting a fundamental genetic process in Huntington’s disease, a rare neurodegenerative disorder.
Cologne-based Disco Pharmaceuticals GmbH has closed its seed financing at €36m. The company will use the proceeds to advance its surfaceome-mapping platform, developing bispecific ADCs and T-Cell Engagers for hard-to-treat cancers.
The US Roche unit Genentech Inc has signed a US$400 million licence deal with British NativeMS specialist OMass Therapeutics Ltd. Under the exclusive collaboration and licence agreement, Genentech intends to commercialise therapies for inflammatory bowel disease.
Pilatus Biosciences AG, a Swiss biotechnology company headquartered in Epalinges with operations in the United States and South Korea, has entered into a clinical trial collaboration with Roche AG. As part of the agreement, Roche will provide its PD-L1 immune checkpoint inhibitor atezolizumab for use in a Phase I study evaluating the combination of atezolizumab with Pilatus’s investigational CD36 inhibitor, PLT012, in patients with hepatocellular carcinoma (HCC).
C4X Discovery Holdings Ltd has received a €8m milestone payment from the French pharmaceutical company Sanofi for its preclinical IL-17A inhibitor. This payment is based on a licence agreement from 2021, under which the British company could receive up to €414m, including royalties.
Jena-based organ-on-chip specialist Dynamic42 has inked a strategic partnership with Ohio Lumex Co., Inc. The Ohio-based company, which is an established provider of life science and analytics solutions, will exclusively distribute Dynamic42’s human 3D-sphaeroid-based organ-on-chip models in the U.S. and Canadian markets.
Swedish clinical-stage pharmaceutical company Vicore Pharma has secured SEK100m, supported by existing and new investors. The injection of capital will allow the company to scale the clinical development of its portfolio of therapies in respiratory and fibrotic diseases.
By Jeremy Levin, D.Phil, MB BChir
Danish AI expert Francesco Pesce and collegues have expanded the toolbox for computational protein design. Their new algorithm will facilitate the design of proteins whose functions exploit the many properties afforded by protein disorder.


 BioDlink
BioDlink Photo from Shawn Day on Unsplash
Photo from Shawn Day on Unsplash  adobe stock photos - deemerwha
adobe stock photos - deemerwha adobe stock photos - Sundry Photography
adobe stock photos - Sundry Photography 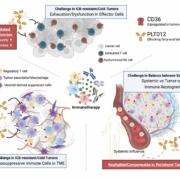 Pilatus Biosciences
Pilatus Biosciences C4X Discovery
C4X Discovery Dynamic42 GmbH
Dynamic42 GmbH AI via Freepik
AI via Freepik Ovid Therapeutics - Jeremy Levin
Ovid Therapeutics - Jeremy Levin marquetand - pixapay.com
marquetand - pixapay.com