Unexpected changes at the top of Rentschler Biopharma: CEO Benedikt von Braunmühl and the Supervisory Board agreed on a swift separation in January, and COO Christiane Bardroff is also set to leave the biopharma manufacturer based in Laupheim, Baden-Württemberg, in the near future. The message from within the company: stay calm, it won´t knock us off course.
ADVERTISEMENT
Tag Archive for: biomanufacturing
Fresenius Kabi and TQ Therapeutics have entered into a strategic development agreement in cell and gene therapy. Fresenius Kabi receives an exclusive licence to develop, manufacture and distribute products incorporating TQ’s cell selection technology. The aim is to enable more scalable, efficient cell therapy production and broader, more cost-effective patient access.
Switzerland is a key hub for biomanufacturing, with immunological products like antibodies
and vaccines making up 18.5% of 2024 exports. Swiss companies drive biotech innovation and biocatalysis
across sectors globally, extending the traditional focus on pharmaceuticals to fine chemicals, fragrances
and food tech. The development is supported by national collaborations and international coalitions.
Biosimilar major Sandoz has started construction of an additional US$440m biosimilar facility near Ljubljana and it set to expand its investment footprint in Slovenia by US$1.1bn.
BioSpring GmbH has broken found for one of the world’s largest production facilities for pharmaceutical nucleic acid-based therapeutics. The new high-tech plant will significantly expand the company’s manufacturing capacity for RNA- and DNA-based active pharmaceutical ingredients, and will support Good Manufacturing Practice (GMP)-compliant production for large-scale projects on behalf of BioSpring’s global partners.
With a 7.7% lead, the incoming Christian Democrat Chancellor Friedrich Merz has put the radical right-wing AfD in its place. Before forming a government in Germany, Merz emphasised that Germany wants to strengthen the EU’s self-sufficiency, although the role of biotechnology seems to be of secondary importance to him.
Rentschler Biopharma announces largest single investment in the higher double-digit range at its headquarters in Germany for construction of a new buffer media facility in Laupheim.
Biomanufacturers must balance the development of new therapeutic modalities and reduce manufacturing costs while maintaining environmental responsibility. Efforts are centred on improving process robustness and efficiency and creating more flexible manufacturing facilities that can accommodate diverse biopharmaceutical products.
If you want to successfully guard against future pandemics, you’re going to need a good plan – and the right kind of vaccine producers. WACKER and CordenPharma have been commissioned by the German government to prepare their mRNA vaccine production lines for a new pandemic.
With the opening of a production centRE for RNA vaccines and therapeutics, German CDMO Wacker Biotech is expanding its expertise in the field of RNA active ingredients.


 Rentschler Biopharma SE
Rentschler Biopharma SE TQ Therapeutics GmbH
TQ Therapeutics GmbH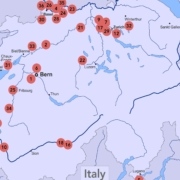 https://www.chimia.ch/chimia/article/view/2025_292
https://www.chimia.ch/chimia/article/view/2025_292 Sandoz
Sandoz BioSpring
BioSpring CSU
CSU Rentschler Biopharma SE
Rentschler Biopharma SE Tosoh Bioscience
Tosoh Bioscience WACKER
WACKER