According to a new deal, AGC Biologics wil manufacture AAVantgarde’s dual AAV gene therapies for inherited retinal diseases using the high-yield BravoAAV™ suspension platform.
ADVERTISEMENT
Tag Archive for: partnership
Boehringer Ingelheim has licenced global rights for CDR111, an antibody-based trispecific M-gager to treat autoimmune diseases from CDR-Life, Inc. CDR111 is an antibody-based T-cell engager designed to selectively target and deplete B cells, with the goal of achieving immune system reset.
CSL has signed an option and collaboration agreement with VarmX valued at up to US$2.2bn. The company will pay US$117m upfront and will fund a global Phase III trial of VMX-C001.
Monte Rosa Therapeutics Inc, founded in Basel in 2018, can look forward to an upfront payment of US$120m for its second collaboration and licensing deal with Novartis AG to develop protein degraders. A total of US$5.7bn is on the table for the new development of an anti-inflammatory candidate that is not part of Monte Rosa’s pipeline.
In a US$450m deal with UK-based Kaerus Bioscience Ltd, Servier SA has acquired the development and commercialisation rights to KER-0193, a Phase II-ready drug candidate for the treatment of rare Fragile X syndrome, the most common monogenic cause of autism.
Polpharma Biologics SA and MS Pharma SA have signed licensing agreements for Polpharma’s vedolizumab (PB016), ocrelizumab (PB018) and guselkumab (PB019) biosimilars in the Middle East and North Africa (MENA) region.
After Finnish protein powder producer Solarfoods signed a strategic agreement with two US food manufacturers to supply 6,000 tonnes of Solein from 2030 onwards, the company has now announced a more concrete partnership with US flavour manufacturer Sensapure Flavours Inc.
California oral macrocyclic peptide specialist Unnatural Products, Inc. and Dutch-Belgian Argenx SE have formed a multi-target research collaboration aimed at developing oral macrocyclic peptide drugs with UNP’s synthetic macrocycle platform.
British cell and gene therapy CDMO eXmoor Pharma plc, and KU Leuven which has just launched a translational cell and gene therapy hub in Belgium have inked a strategic collaboration supporting the hub’s work in four areas.
Building on the January 2025 ALS drug discovery collaboration with Alchemab Therapeutics, Eli Lilly & Company has secured rights to the first IND-ready program under a US$415m licensing agreement.


 stock.adobe.photo.com/Popelniushka
stock.adobe.photo.com/Popelniushka  Boehringer Ingelheim
Boehringer Ingelheim Adobe stock photos - nadia
Adobe stock photos - nadia Rosa Therapeutics Inc
Rosa Therapeutics Inc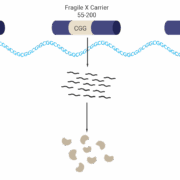 FRAXA Ewsearch Organisation
FRAXA Ewsearch Organisation Polpharma Biologics SA
Polpharma Biologics SA Solar Foods
Solar Foods Unnatural Products Inc
Unnatural Products Inc adobe stock - aliaksandr
adobe stock - aliaksandr