BioNTech: ADC study continues with conditions
Good news for BioNTech SE: The safety study of the ADC BNT326/YL202 developed by MediLink Therapeutics, which was suspended by the FDA in mid-June, will continue.
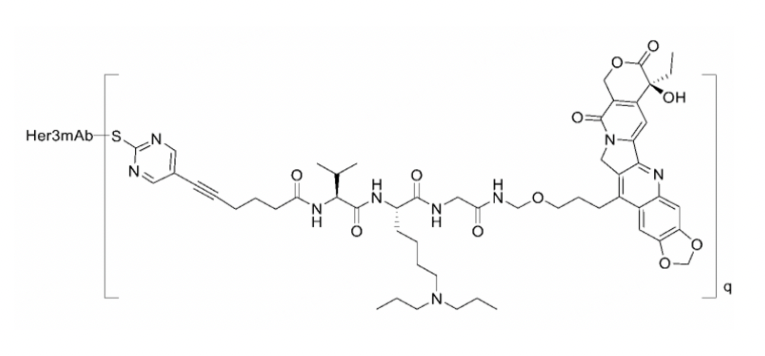
The FDA’s suspension of the open-label Phase I dose-finding study of the antibody-drug conjugate (ADC) BNT326/YL202 (NCT05653752) developed by MediLink Therapeutics (Suzhou) Co., Ltd. due to three ADC-related out of 14 deaths has been lifted. However, the US regulatory authority has limited the therapeutic window: The drug concentration of BNT326/YL202 must not exceed 3mg/kg body weight. The ADC, which is directed against the human epidermal growth factor receptor 3 (HER3), is being developed by BioNTech SE and Chinese MediLink. Patients will again be enrolled in the trial. In the dose levels below 3 mg/kg, “a controllable safety profile with encouraging clinical activity was observed”, the Mainz-based company stated.
The study sponsor MediLink had observed a dose-dependent trend in treatment-related adverse events for BNT326/YL202. This related in particular to a decrease in neutrophils (neutropenia). In addition, an increasing rate of mucositis was observed. These events are frequent TRAEs of established chemotherapies and increase the risk of developing serious infections. Neutropenia might be controlled by dose reduction, dose interruption or administration of recombinant granulocyte colony-stimulating factor.
Accordingly, the companies must refrain from enrolling further patients in dose cohorts of more than 3 mg/kg. In parallel, the investigator’s brochure, informed consent form and clinical study protocol will be updated with revised guidance on dose delay, reduction and adjustment and on the administration of prophylactic medication against TRAEs.
In view of three treatment-related deaths in the unblinded Phase I dose-finding study (2 in the 4 mg/kg, one in the 5.5 mg/kg group), the FDA had temporarily stopped the study in mid-June due to potential dose-limiting toxicity. Data presented at the ASCO meeting 2024 showed a response rate of 54 patients enrolled by February 2024 of 91%, and one complete response. In presentations, MediLink tells that the ADC constructed from an anti-HER3 antibody, a protease-sensitive linker and a camptothecin topoisomerase I blocker is cleaved extracellularly in the tumour microenvironment and thus the payload concentration is higher there because its release is independent of the uptake into lysosomes. According to the Human Protein Atlas HER3 (ERBB2, LCCS2) not very tumour specific. Experts, however, told European Biotechnology that MediLink’s ADC show differences concerning the payload strucure and the linker to to Daiichi Sankyo‘s Phase III Her3-ADC patritumab deruxtecan, which might have triggered the safety issues. It is now to be seen, if a dose of 3mg/kg BNT326/YL202 will show efficacy in patients with colorectal carcinoma or other solid tumours.


 BioNTech SE
BioNTech SE Araris Biotech AG
Araris Biotech AG Lonza Group
Lonza Group