Egetis Therapeutics has submitted a rolling NDA for Tiratricol to treat rare MCT8 transporter deficiency. The application for priority review is based on positive ReTRIACt study results.
ADVERTISEMENT
Tag Archive for: FDA
Amsterdam-based UniQure NV’s stocks made a 240% after-hours jump at Nasdaq after the company announced its Huntington gene therapy AMT-130 met the endpoints of a Phase II study and it will apply for marketing authorisation next year. In addition, UniQure entered into a US$175m non-dilutive senior secured term loan facility with Hercules Capital and commenced a US$200m underwritten public offering of its ordinary shares to prepare a BLA in Q1 2026.
The FDA under President Trump is heading for a radical break with decades of practice: the external advisory committees that have long served as critical checks in approval decisions are to be largely sidelined. George Tidmarsh, head of the Centre for Drug Evaluation and Research, has dismissed the panels as redundant and instead wants to rely on the publication of Complete Response Letters (CRLs). Officially, the move is framed as efficiency – in reality, it raises serious questions about the agency’s transparency.
French targeted DNA gene therapy specialist EG427 has closed a €27m Series B financing co-led by VC investor Andera Partners and BPI France to initiate Phase I testing of a Herpes Simplex Virus 1 (nrHSV-1) vector that specifically targets type C neurons to suppress dysfunctions of the bladder in people with spinal injury.
After meeting the primary endpoints of a pivotal Phase II trial, UK-based Adaptimmune Ltd. is submitting an FDA Biologics License Application for its autologous T-cell receptor therapy (TCR-T) Lete-cel (letetresgene autoleucel).
Good news for BioNTech SE: The safety study of the ADC BNT326/YL202 developed by MediLink Therapeutics, which was suspended by the FDA in mid-June, will continue.
German AdrenoMed AG has got FDA Fast Track designation for its targeted septic shock medicine enibarcimab that restores endothel integrity.
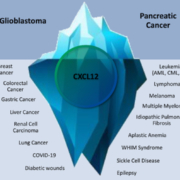
TME Pharma (France/Germany) announced that the US Food and Drug Administration (FDA) has granted Fast Track designation for NOX-A12 (olaptesed pegol), TME Pharma’s CXCL12 inhibitor, in combination with radiotherapy and bevacizumab for use in the treatment of the aggressive adult brain cancer glioblastoma.
Following in the footsteps of the European Medicines Agency (EMA), the US FDA is now also investigating the side effects of obesity blockbusters.


 FDA
FDA uniqure
uniqure  FDA
FDA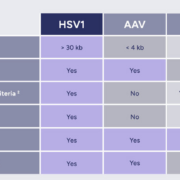 EG 427
EG 427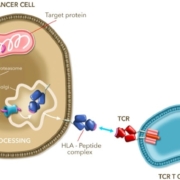 Adaptimmune
Adaptimmune Medilink Therapeutics
Medilink Therapeutics