The European Medicines Agency (EMA) and the US Food and Drug Administration (FDA) have together compiled ten principles for good artificial intelligence (AI) practice in drug development.
ADVERTISEMENT
Tag Archive for: Regulatory
As the regulatory strategy becomes a boardroom-level concern for biotech executives pursuing global expansion, knowing how the Saudi Food and Drug Authority (SFDA) and the European Medicines Agency (EMA) differ is crucial, particularly as Saudi Arabia accelerates its ambition to become a global life sciences hub. Although both regulators adhere to international norms, their differences in review speed, procedural flexibility, and strategic positioning can have a direct impact on competitive advantage, investment planning, and time to market.
Egetis Therapeutics has submitted a rolling NDA for Tiratricol to treat rare MCT8 transporter deficiency. The application for priority review is based on positive ReTRIACt study results.
The European Commission has launched a public consultation to support the development of the European Biotech Act, which is now being prioritised after a previous delay. The Commission plans to publish the legislative proposal by the end of 2025 and is inviting stakeholders across the biotechnology sector to provide feedback on regulatory, financial, and operational barriers.
In a surprise move, Health Commissioner Oliver Várhelyi announced at the Medicines for Europe Annual Conference that the EU Biotech Act will go ahead this year, reversing its provious delay from Q3 2025 to Q3 2026. The legislation has now been fast-tracked because the Commission recognised biotechnology’s rising geostrategic weight.
The EMA has asked the industry to share their thoughts on its draft guidelines for developing and assessing biosimilars. The idea is to make it easier for patients in the EU to get access to biosimilars, while also keeping Europe a good choice for developers.




 FDA
FDA adobe stock photos - ndomble
adobe stock photos - ndomble  Medicines for Europe
Medicines for Europe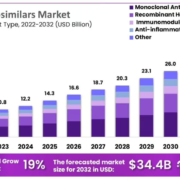 market.us
market.us