Zurich-based oncology biotech Araris Biotech AG has entered into a research collaboration with an option to license with Japan’s Chugai Pharmaceutical, a subsidiary of Roche. The aim of the partnership is to develop next-generation antibody–drug conjugates (ADCs). While Araris itself is now Japanese-owned – a wholly owned subsidiary of Taiho Pharmaceutical, part of the Otsuka Group – its research activities remain firmly rooted in Zurich. That presence was highlighted last year when the Strüngmann brothers, German billionaire twins, awarded a prize to the company’s founders.
ADVERTISEMENT
Tag Archive for: ADC
CDMOs are currently setting the agenda across the industry. Alongside peers such as Vetter, Lonza has now delivered a clear statement of intent: strong results, sharper focus and an even more determined commitment to its core business. But the stronger investment strategy internationally frightens Switzerland – at least some analysts.
Positive signals from a partnership with Takeda are allowing Heidelberg Pharma to push the setback from a milestone payment not received as planned from Telix somewhat into the background. Nevertheless, the company has been strongly shaken and is now hoping for calmer waters in order to drive its clinical development forward with greater focus.
QLi5 Therapeutics has completed a €6.26 million capital increase led by its Korean co-founder, Qurient, which has also increased its voting stake in the German biotech. The funding will be used to advance QLi5’s proteasome inhibitor(PI)-based antibody-drug conjugate (ADC) payload platform, an approach the company is presenting as a next step beyond today’s dominant ADC payload classes.
For BioNTech, 2026 could become one of the most decisive years since its pandemic-driven breakthrough. The pipeline is broad, the coffers are well stocked, partnerships are numerous, and the outlook extends far beyond 2030. Yet despite all the strategic preparation, one key question will be answered in the coming months: can the clinical data live up to the high expectations surrounding BioNTech’s cancer strategy?
Roche is back in China — and this time at a very different price point. In a renewed partnership with MediLink Therapeutics, the Swiss pharmaceutical group has secured a second antibody–drug conjugate (ADC), underlining how the first collaboration has paid dividends for both sides.
Antibody-drug conjugates (ADCs) hinge on the antibody, not merely the payload or linker. Antibody precision defines efficacy, safety and therapeutic window. Emerging antibody formats – bispecifics, conditional designs and TCR-mimics – expand target space, demanding rigorous engineering to realise next-generation ADC potential.
Solid tumours pose major obstacles for cell therapies, but German-American biotech T-knife is tackling them head-on. CEO Tom Soloway and CTO Elisa Kieback share how their “supercharged” TCR-Ts are engineered to overcome these challenges.
Following its US$361 m Series C financing, Tubulis AG presented first Phase I/IIa data on its lead ADC, TUB-040, at ESMO 2025 in Berlin. The study focuseds on patients with platinum-resistant ovarian cancer (PROC). TUB-040 shows clear differentiation potential
French ADC start-up Adcytherix SAS has added €105m Series A funding to a €30m seed financing round closed last year. With the money, the company will start a Phase I study with its lead ADCX-020 in Q1 2026 and develop novel payloads to break ADC therapy resistance.
Tag Archive for: ADC
The 16th World ADC London takes place on 23–26 February 2026, bringing together over 800 senior decision-makers from biopharma, KOL oncologists, service providers, investors, and regulators. As Europe’s largest forum dedicated to antibody–drug conjugates (ADCs), the summit offers a comprehensive programme covering discovery, translational science, clinical development, manufacturing, and regulatory strategy.
ADCs continue to transform oncology, delivering potent therapies directly to cancer cells while minimising off-target toxicity. As the field moves towards earlier-line treatments, developers face complex challenges, from payload optimisation and resistance mechanisms to scalable manufacturing. World ADC London 2026 addresses these challenges head-on, providing actionable insights from 110 global speakers across five content streams over four days of in-depth sessions, workshops, and panel discussions.
This year’s programme highlights next-generation payloads, bispecific constructs, dual-payload strategies, and non-antibody conjugates, giving attendees practical guidance on overcoming resistance, improving safety, and navigating evolving regulatory expectations. Strategic and commercial considerations are also central, with presentations from leading clinicians, regulatory experts, and investors sharing insights into clinical advancement, partnership opportunities, and emerging trends shaping the ADC landscape.
Networking remains a cornerstone of the event, with 14 hours of dedicated interaction built into the agenda. Structured networking sessions, one-to-one meetings, and informal discussions enable delegates to forge collaborations, explore licensing and investment opportunities, and exchange knowledge with peers from across the global ADC community.
Whether you are an early-career scientist, an experienced developer, or a strategic investor, World ADC London 2026 is the must-attend event for staying at the forefront of ADC innovation. From cutting-edge science to strategic insights and invaluable industry connections, the summit empowers participants to advance the next generation of ADCs from the lab to clinical and commercial success.
Contact: info@hansonwade.com | Event Guide | Registration & Pricing


 Araris Biotech AG
Araris Biotech AG Lonza Group
Lonza Group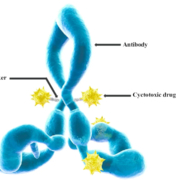 International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
International Journal of Molecular Sciences, doi: 10.3390/ijms17040561 JO - s
 BioNTech SE
BioNTech SE Roche
Roche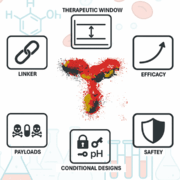 Yumab GmbH
Yumab GmbH Die Hoffotografen GmbH
Die Hoffotografen GmbH BIOCOM Interrelations
BIOCOM Interrelations Adcytherix SAS
Adcytherix SAS