Depending on who you talk to, artificial intelligence (AI) is either a saviour or an enslaver. The concerns range from loss of jobs through to robots running society. However, in the life sciences, the use of AI and machine learning (ML) have created some excitement over their potential applications.
ADVERTISEMENT
Conventional meat production contributes to 15% of the global greenhouse gas emissions, high energy consumption and land use change. Belgian Paleo NV produces alternative protein from myoglobins of different species.
Generic and biosimilar manufacturer Sandoz is expanding with a new biologics production facility in Lendava, Slovenia to prepare for more demand in Europe for biosimilar products.
The Sweden Biobank Act governs the collection, storage, and usage of identifiable human material samples for specific purposes.
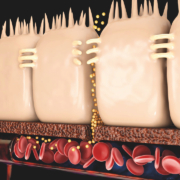
Alentis is the only company developing potential treatments for solid cancers and fibrosis targeting Claudin-1 (CLDN1). The molecule is significantly involved in the tight junction between cells. Now the company closed a three digit million Seriec C round.
From bacteria to cellulose fibers and lab grown paper. A greek project paves the way to save trees.
Tilos marks the first island to achieve 100% diversion from landfill with an 87% recycling rate.
The new Krakow headquarters of preclinical CRO Selvita have opened.
How to stop the silent pandemic AMR was discussed by 500 experts from 13 countries at the 7th AMR Conference in Basel. At that time, in Brussels, there were two proposals on the table on how to compensate novel resistance-breaking antimicrobials, from which one got the green light in the new pharma legislation.