Polpharma Biologics SA and MS Pharma SA have signed licensing agreements for Polpharma’s vedolizumab (PB016), ocrelizumab (PB018) and guselkumab (PB019) biosimilars in the Middle East and North Africa (MENA) region.
ADVERTISEMENT
Tag Archive for: Biosimilars
Biosimilar major Sandoz has started construction of an additional US$440m biosimilar facility near Ljubljana and it set to expand its investment footprint in Slovenia by US$1.1bn.
The EMA has asked the industry to share their thoughts on its draft guidelines for developing and assessing biosimilars. The idea is to make it easier for patients in the EU to get access to biosimilars, while also keeping Europe a good choice for developers.
AVT03, a biosimilar candidate for osteoporosis treatment, showed promise in treating bone illnesses, according to results from a pharmacokinetic study by Alvotech SA.
Generic and biosimilar manufacturer Sandoz is expanding with a new biologics production facility in Lendava, Slovenia to prepare for more demand in Europe for biosimilar products.
The region regulated by the European Medicines Agency (EMA) has been the most proactive globally in the early adoption of biosimilars. By February 2023, it had approved around 75 products containing them. The distribution of such compounds within Europe, however, has been uneven, with some countries offering more market opportunities than others. Beyond Europe, few producers have a strategy for the Middle East and/or Africa, and India and South Korea could soon jump in to fill the gap.


 Polpharma Biologics SA
Polpharma Biologics SA Sandoz
Sandoz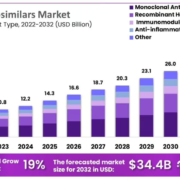 market.us
market.us