Luxembourg-based venture capital specialist Vesalius Biocapital has closed the first round of Biocapital III fund that aims to invest into later-stage European life sciences companies.
ADVERTISEMENT
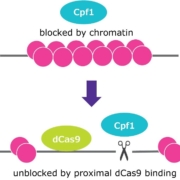
German Merck KGaA has developed a new genome editing tool dubbed proxy-CRISPR providing access to previously inaccessible microenviroments of the genome by modification of chromatin modifications.
Antibody developer Kymab Ltd has instated Sonia Quaratino as its first Chief Medical Officer. She will manage the clinical development of the Group’s expanding therapeutic antibody portfolio.
Astellas Pharma Inc. has completed the acquisition of Ogeda SA (previously Euroscreen SA, Gosselies, Belgium).
Swiss Vifor Pharma has agreed to acquire shares of Cambridge-based distributor Adebia worth US$50m in order exclusivly distribute its hypoxia-inducible factor (HIF) stabiliser vadadustat to 40% of US dialysis clinics.
Small companies and academic institutions develop their assets, in most cases, under budget constraints. Still, they have to meet the expectations of future strategic partners or investors in order to be an attractive option for them and to have a chance to get the product onto the market.
AstraZeneca’s anti PD-L1 antibody durvalumab has met the co-primary endpoint in non-small cell lung cancer (NSCLC) in a Phase III trial vs placebo. OS data were not yet available.
Drug makers have only until two years from now to make their drugs and packaging counterfeit-resistant. By 9 February, 2019, every prescription drug pack must carry a 2D data matrix code that can be tracked by wholesalers and pharmacists along each stage of the value chain. Additionally, each pack must be sealed with an anti-tampering device. If companies and the NMVOs that handle national databases can’t manage the task, their drugs cannot be sold after the deadline.