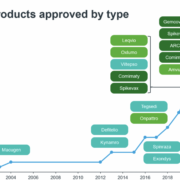
The biologics contract development and manufacturing (CDMO) market is experiencing rapid growth and is dominated by global players with investments in large scale stainless steel capacity.
But is larger always better? Not necessarily says Celonic, a mid-sized, privately owned CDMO, who has been embracing next generation technologies to help small to large biotech customers bring their drugs reliably, effectively and efficiently to the market.
ADVERTISEMENT
Tag Archive for: CDMO
After three and a half years of construction, the CDMO Richter Biologics has inaugurated its new multipurpose production facility that triples the CDMO’s capacity for the bacterial production of pDNA, vaccines, nanobodies and recombinant proteins.
With the opening of a production centRE for RNA vaccines and therapeutics, German CDMO Wacker Biotech is expanding its expertise in the field of RNA active ingredients.
Danish CDMO 21st.BIO A/S has unveiled a new pilot plant facility to accelerate scaling of biotech processes ranging from food and beverages, agriculture, biomaterials, and biopharma.
Professionalism and quality are always at the forefront along the whole value chain: from gene to product. To pro-actively meet the evolving demand of the market to produce new assets Richter-Helm significantly increased its manufacturing capacities for biopharmaceutical products at its production site in Bovenau, Germany.
The biotech and pharma branch is growing steadily. Many companies have full order books for the next few years and expect growth. This is the basis for new investments in assets and infrastructure. These growing structures often expose vulnerabilities and challenges for the business. Therefore, companies need to increase the stability and efficiency of their processes.
Bayer SE subsidiary Asklepios BioPharmaceutical Inc. (AskBio) has started Phase II testing of AB-1002 (NAN-101) in patients with congestive heart failure.
Biovian and 3P Biopharmaceuticals have created a new pan-European CDMO, dubbed 3PBIOVIAN.
When businesses receive a proposal from a contract development and manufacturing organisation (CDMO), it’s much like a first date: first impressions matter. The proposal process not only reveals a CDMO’s services but also hints at the potential relationship’s quality. This is gauged mainly through metrics like timing, quality, and price.
Sotio hsd licenced Synaffix’ ADC platform for up to US$740m to develop up to three next generation bioconjugates.


 2024 Cytiva, Reproduced with Permission of Owner
2024 Cytiva, Reproduced with Permission of Owner BIOCOM Interrelations GmbH
BIOCOM Interrelations GmbH