Watch this expert-led session to explore the latest trends and insights in gene therapy, including how to model immunosuppressive regimens, key study-design considerations, and the importance of age categories in nonhuman primate research.
ADVERTISEMENT
Download the guide to explore how forward-thinking organisations are leveraging digital transformation in manufacturing to overcome regulatory challenges, enhance compliance, and gain operational efficiencies across multiple European sites and jurisdictions.
Customized Enzymes and Reagents from New England Biolabs. Creating the right partnership is essential when pioneering a new life science product.
In biomedical research and biomanufacturing, the purity of reagents directly impacts the reliability of experimental outcomes and the safety of downstream therapeutic products. Endotoxins are notorious contaminants in protein preparations. Even trace amounts of endotoxin in recombinant proteins can trigger potent immune responses, distort results, and compromise patient safety, especially in sensitive applications such as immunology, cell and gene therapy, and vaccine production. Sino Biological has addressed this urgent need with the launch of ProPure™, an advanced line of endotoxin-free recombinant proteins.
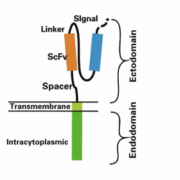
CAR-T cell therapy has demonstrated remarkable success in treating hematologic malignancies and is now expanding into solid tumors. On June 1, 2025, The Lancet published positive results from the world’s first randomized controlled trial of CLDN18.2 specific CAR-T in gastric cancer, led by Professor Lin Shen’s team at Peking University.
Stick, Tag, Destroy: The Science of PROTACs and MGDs – For a long time, traditional drug research focused on inhibitors that block the active sites of disease-related proteins. However, around 80% of these proteins remain “undruggable” because they lack clear binding sites.
POWTECH TECHNOPHARM (Nuremberg, 23-25 September 2025) is the international trade fair for process engineering experts and a tailor-made technology platform for the pharmaceutical and life science industries.
Chikungunya virus (CHIKV) is a mosquito-borne alphavirus causing fever, severe joint pain, rash, and muscle aches, mainly transmitted by Aedes mosquitoes. The outbreak began on islands in the Indian Ocean and had spread to 119 countries by 2025, including temperate regions such as France. Key control strategies include vector control, surveillance, health system preparedness, and international cooperation. Sino Biological supports research by providing recombinant CHIKV antigens and antibodies, which are useful for vaccines, antiviral drugs, and diagnostics.
Sino Biological US, Inc. proudly unveils ProPure™, an industry-leading line of ultra-pure, endotoxin-free recombinant proteins, fully produced in the USA at its state-of-the-art Center for Bioprocessing (C4B) facility in Houston, Texas.
Iions.bio was founded in 2024 and is located in the Innovation and Startup Centre IZB in Planegg-Martinsried.






 Sino Biological
Sino Biological
 Sino Biological
Sino Biological Sino Biological, Inc.
Sino Biological, Inc. ions.bio
ions.bio