Sino Biological US, Inc. proudly unveils ProPure™, an industry-leading line of ultra-pure, endotoxin-free recombinant proteins, fully produced in the USA at its state-of-the-art Center for Bioprocessing (C4B) facility in Houston, Texas.
ADVERTISEMENT
Tag Archive for: Sino Biologicals
Antibody-Drug Conjugates (ADCs) have been transformative in oncology, offering targeted cancer treatments that enhance efficacy while sparing healthy cells. However, the potential of ADCs extends well beyond cancer care. From autoimmune diseases to metabolic disorders, advancements in ADC technology are paving the way for breakthroughs across a range of therapeutic areas. This article explores how ADCs are redefining treatment paradigms and improving patient outcomes in diverse fields of medicine.
Antibody-drug conjugates (ADCs) are a new class of biotherapeutics, consisting of acytotoxic payload covalently bound to an antibody by a linker. Evaluating the pharmacokinetics (PK) properties of ADCs in preclinical and clinical studies is essential for their strategic design and successful development.
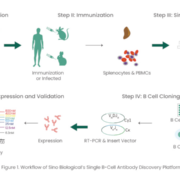
Since the approval of Orthoclone OKT3 in 1986, more than 100 monoclonal antibodies (mAbs) have been approved by the Food and Drug Administration (FDA) to treat a variety of diseases ranging from autoimmune disorders, infectious diseases, and cancer [1-2]. In the context of the current COVID-19 pandemic, it´s crucial that these biologics are developed rapidly and efficiently. Among various antibody discovery approaches, including hybridoma technology, single B cell screening is a powerful and efficient strategy for generating antigen-specific mAbs based on the direct amplification of the VH and VL regions encoding genes from single B cells [3-4]. Notably, single B cell screening has various advantages that include maintaining the naïve VH/VL pairing, requiring relatively few cells, and the ability to discover antibodies against challenging targets.


 Sino Biological, Inc.
Sino Biological, Inc. Sino Biological
Sino Biological