Switzerland is a key hub for biomanufacturing, with immunological products like antibodies
and vaccines making up 18.5% of 2024 exports. Swiss companies drive biotech innovation and biocatalysis
across sectors globally, extending the traditional focus on pharmaceuticals to fine chemicals, fragrances
and food tech. The development is supported by national collaborations and international coalitions.
ADVERTISEMENT
Spain has once again secured the top position in Europe for clinical trials. According to various Clinical Research Organisations at BioSpain 2025 (7–9 October, Barcelona), this is largely due to the speed with which the Spanish Medicines Agency (AEMPS) approves clinical studies.
The EMBL Imaging Centre has received €7 million in support from Boehringer Ingelheim Stiftung to further advance its cutting-edge imaging technologies.
Biomanufacturing promises domestic, sustainable, sovereign and resilient production. Capacities are trapped in outdated scale-up processes. SPRIND urges investment and disruptive innovation to propel biomanufacturing. Demands are decentralised, non-sterile & continuous biomanufacturing capabilities.
Resilience and quality connections define Chemspec Europe 2025.
A landmark study in the UK shows that mitochondrial replacement therapy can prevent babies from inheriting diseases caused by mutant mitochondria.
That deserves a closer look: The recently announced partnership between Axxam S.p.A., a leading provider of integrated early discovery services, and Molecular Health GmbH, a pioneer in AI-powered data solutions for pharmaceutical R&D, is more than a standard collaboration. It exemplifies the convergence of consolidated in-vitro biology-led drug discovery with modern, data-intelligent drug development methodologies. As an example for breaking the silos of clinical and preclinical target validation.
Spain’s biotech sector drives innovation, economic growth, and scientific excellence. With strong R&D investment, global research impact, and a leading role in gender equality, it’s a key player in health, sustainability, and strategic autonomy. BIOSPAIN 2025 in Barcelona will showcase its potential and connect global biotech leaders.
Pichia pastoris (syn. Komagataella phaffii), a methylotrophic yeast, is a proven platform for pharmaceutical protein production. However, its potential reaches far beyond. As a reliable, scalable, and cost-efficient platform for recombinant protein expression, Pichia is equally suited for industrial enzymes, food & feed, diagnostics, and biomaterials.
In today’s rapidly evolving world, adapting to demand volatilities while ensuring uninterrupted supply of biological medicines is more critical than ever. At Boehringer Ingelheim, our global biopharmaceutical network delivers flexibility, resilience, and reliability. By leveraging harmonised procedures, standardised setups, and innovative logistics, we ensure the biologics we produce reach those in need – even during unexpected challenges.


 https://www.chimia.ch/chimia/article/view/2025_292
https://www.chimia.ch/chimia/article/view/2025_292 AseBio
AseBio Kinga Lubowiecka/ EMBL
Kinga Lubowiecka/ EMBL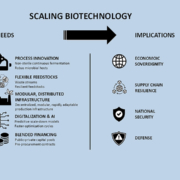 SPRIND
SPRIND
 MedicalWorks from Adobe Stock
MedicalWorks from Adobe Stock Axxam S.p.A
Axxam S.p.A ASEBIO
ASEBIO Validogen GmbH
Validogen GmbH Boehringer Ingelheim Biopharmaceuticals GmbH
Boehringer Ingelheim Biopharmaceuticals GmbH