With the promise of transformative treatments, cell therapies are rising across oncology, regenerative medicine, and stem cell applications. PL BioScience supports this evolution with Human Platelet Lysate (HPL) and soon, the first artificial HPL, while expanding production in Aachen to continue to deliver this high-quality cell culture supplement worldwide.
ADVERTISEMENT
One year after the inauguration of Bayer Co.Lab — the open workspace for start-ups in Berlin — it is time to take stock. European Biotechnology News Magazine
spoke with tenants and with Dr Ruth Shah, Head of Bayer Co.Lab Berlin, about the concept, the offering, and the feedback Bayer has received so far.
Antibody-drug conjugates (ADCs) hinge on the antibody, not merely the payload or linker. Antibody precision defines efficacy, safety and therapeutic window. Emerging antibody formats – bispecifics, conditional designs and TCR-mimics – expand target space, demanding rigorous engineering to realise next-generation ADC potential.
Earlier guidelines such as ICH Q2(R1) and USP <1224>, <1225>, and <1226> viewed analytical method validation as a discrete activity focused primarily on chromatographic procedures. With ICH Q14, ICH Q2(R2), and USP <1220>, a life-cycle approach now considers variability and improves reliability, supported by tools like DoE, multivariate statistics, and digital technologies enabling Quality by Design and risk-based systems.
In Life Sciences, SAP transformations for midsize CDMOs are nothing short of complex. But integrating a newly acquired site into an existing SAP landscape is a different matter entirely. At Tenthpin, we took a bold step by designing a new SAP S/4HANA template from the ground up to a new site for a leading CDMO; and then successfully deploying it to another newly acquired site in less than 10 months.
Essential areas such as study planning, interpretation, regulatory guidance, and key study design decisions remain human responsibilities, although AI increasingly supports routine tasks. X-act gets involved after the preclinical phase supporting clients in all clinical research phases from Phase I to IV and beyond. Their service ranges from initial planning to final analysis, reporting, and regulatory submission. European Biotechnology spoke with Jasmin Atarodi, Managing Director of X-act Cologne Clinical Research GmbH, about the benefits of partnering with experienced data specialists.
Solid tumours pose major obstacles for cell therapies, but German-American biotech T-knife is tackling them head-on. CEO Tom Soloway and CTO Elisa Kieback share how their “supercharged” TCR-Ts are engineered to overcome these challenges.
New developments under the current U.S. administration may be a challenge for the pharma/biotech industry: the Inflation Reduction Act, Orange Book Challenges, Most Favored Nation Policy, and the new Trump-Lutnick Patent Tax. And it will likely affect European pharma and biotech.
Out of 43 pitches, the founders of Spanish Opportunity Health S.L. delivered the best presentation for a new life sciences product: a choking device that can potentially be used by anyone in emergency situations without the need for external assistance.
Alongside a start-up pitch featuring 45 international participants, the second day of BioSpain 2025 (7–9 October 2025, Barcelona) offered biotech start-ups a panel discussion with prominent US investors, who shared their selection criteria and outlined the impact of the current geopolitical situation on investment decisions.


 Alexander Sievert Photography
Alexander Sievert Photography  Bayer Co.Lab
Bayer Co.Lab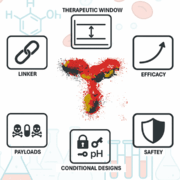 Yumab GmbH
Yumab GmbH Concept Heidelberg GmbH
Concept Heidelberg GmbH Tenthpin
Tenthpin X-act Cologne Clinical Research GmbH
X-act Cologne Clinical Research GmbH Die Hoffotografen GmbH
Die Hoffotografen GmbH Boehmert & Boehmert
Boehmert & Boehmert AseBio
AseBio  AseBio
AseBio