Roche withdraws checkpoint blocker from the market
Roche AG's US subsidiary Genentech Inc has withdrawn its checkpoint inhibitor atezolizumab from the market after disappointing results in bladder cancer.
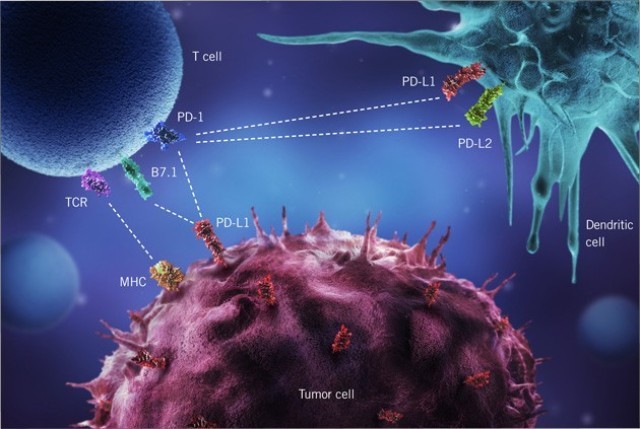
Switzerland’s Hoffmann La Roche AG no longer markets its cancer immunotherapy atezolizumab (Tecentriq) for the treatment of urothelial carcinoma in the USA: The reason for the pharmaceutical company’s voluntary withdrawal from the largest western pharmaceutical market is the disappointing results of a post-marketing study that the Food and Drug Administration (FDA) had imposed on the manufacturer before the final marketing approval. Because of the medically high need for effective bladder cancer therapies, the FDA had initially granted Roche/Genentech accelerated approval on a thin data basis. The marketing authorisation remains valid for the indications lung cancer, breast carcinoma and liver carcinoma.
Atezolizumab binds to the surface protein PD-L1 (“programmed cell death 1 ligand 1”), with which cancer cells protect themselves from attack by the immune system, and was approved six years ago as a second-line therapy after cisplatin-based chemotherapy failed or could not be administered. Even then, the results were not very convincing; partial remission was achieved in only 14.8% of patients.
The follow-up study IMvigor130 with 1,214 participants worldwide, in which alezolizumab was administered together with gemcitabine and carboplatin/cisplatin and compared with chemotherapy, however, did not deliver results even in patients who expressed a lot of PD-L1: Their overall survival did not increase significantly.
After the withdrawal in the USA, it should not be long before the checkpoint inhibitor is also withdrawn from the market in Europe.


 Immunic/Nela Dorner
Immunic/Nela Dorner