The FDA under President Trump is heading for a radical break with decades of practice: the external advisory committees that have long served as critical checks in approval decisions are to be largely sidelined. George Tidmarsh, head of the Centre for Drug Evaluation and Research, has dismissed the panels as redundant and instead wants to rely on the publication of Complete Response Letters (CRLs). Officially, the move is framed as efficiency – in reality, it raises serious questions about the agency’s transparency.
ADVERTISEMENT
Tag Archive for: market approval
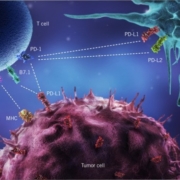
Roche AG’s US subsidiary Genentech Inc has withdrawn its checkpoint inhibitor atezolizumab from the market after disappointing results in bladder cancer.


 FDA
FDA