Zurich-based oncology biotech Araris Biotech AG has entered into a research collaboration with an option to license with Japan’s Chugai Pharmaceutical, a subsidiary of Roche. The aim of the partnership is to develop next-generation antibody–drug conjugates (ADCs). While Araris itself is now Japanese-owned – a wholly owned subsidiary of Taiho Pharmaceutical, part of the Otsuka Group – its research activities remain firmly rooted in Zurich. That presence was highlighted last year when the Strüngmann brothers, German billionaire twins, awarded a prize to the company’s founders.
ADVERTISEMENT
Tag Archive for: Roche
Roche made a clear statement in 2025. The Basel-based group increased sales by 7% at constant exchange rates to CHF 61.5bn (€67bn) and lifted core operating profit by as much as 13%. For many observers, this represents far more than just a solid set of annual results.
For BioNTech, 2026 could become one of the most decisive years since its pandemic-driven breakthrough. The pipeline is broad, the coffers are well stocked, partnerships are numerous, and the outlook extends far beyond 2030. Yet despite all the strategic preparation, one key question will be answered in the coming months: can the clinical data live up to the high expectations surrounding BioNTech’s cancer strategy?
Roche is back in China — and this time at a very different price point. In a renewed partnership with MediLink Therapeutics, the Swiss pharmaceutical group has secured a second antibody–drug conjugate (ADC), underlining how the first collaboration has paid dividends for both sides.
Roche has unveiled detailed data for its oral selective estrogen receptor degrader (SERD), giredestrant, at the 2025 San Antonio Breast Cancer Symposium.
Roche has reported that its selective estrogen receptor degrader (SERD), giredestrant, has demonstrated significant clinical benefit in pivotal Phase III trials for adjuvant early-stage ER-positive, HER2-negative breast cancer, as well as strong efficacy in advanced breast cancer settings. These results position giredestrant as the first oral SERD to show meaningful clinical benefit in this indication. The announcement sparked a notable 7% jump in Roche’s share price in a single day.
Roche Diagnostics and its partner KlinRisk Inc have received the EU CE mark for the very first AI-based risk stratification tool that reliably assesses progressive decline in kidney function.
The U.S. FDA has approved atezolizumab and atezolizumab with hyaluronidase in combination with lurbinectedin as the first and only first-line maintenance therapy for adults with extensive-stage small cell lung cancer (ES-SCLC) whose disease has not progressed after induction therapy with atezolizumab, carboplatin and etoposide.
Roche has signed a definitive merger agreement to acquire 89bio and its Phase III candidate pegozafermin, an FGF21 analogue for the treatment of moderate to severe metabolic dysfunction-associated steatohepatitis (MASH). The deal is expected to close in Q4 2025.
Data on the primary endpoint of BioNTech’s/Duality Biologics’ pivotal Phase III study of its HER2 antibody-drug conjugate BNT323/DB-1303 are not yet public, However, BioNTech says that progression-free survival is better than with Roche’s second-line breast cancer therapy Kadcyla.


 Araris Biotech AG
Araris Biotech AG Roche
Roche BioNTech SE
BioNTech SE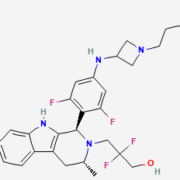 PubChem
PubChem Roche
Roche Roche Diagnostics International
Roche Diagnostics International PharmaMar SA
PharmaMar SA Henk Vrieselaar - stock.adobe.com
Henk Vrieselaar - stock.adobe.com  Marcus Krauss - stock.adobe.com
Marcus Krauss - stock.adobe.com