Lisbon’s immuno-oncology company Mondego Bio Lda has left stealth mode through a Series A financing led by Biovance Capital, supported by OrbiMed and Torrey Pines.
ADVERTISEMENT
Due to its properties, plastic is a sought-after material for medical technology components. Depending on the specification, the manufacturing of these components is subject to strict hygiene measures. The whitepaper from RKT shows what is important in plastic injection molding and the assembly of components under cleanroom conditions.
Roche has signed a definitive merger agreement to acquire 89bio and its Phase III candidate pegozafermin, an FGF21 analogue for the treatment of moderate to severe metabolic dysfunction-associated steatohepatitis (MASH). The deal is expected to close in Q4 2025.
CSL has signed an option and collaboration agreement with VarmX valued at up to US$2.2bn. The company will pay US$117m upfront and will fund a global Phase III trial of VMX-C001.
British Enhanced Genomics Ltd has extended its Series A funding to US$19m. Investors include BGF, Parkwalk, and Meltwind.
Monte Rosa Therapeutics Inc, founded in Basel in 2018, can look forward to an upfront payment of US$120m for its second collaboration and licensing deal with Novartis AG to develop protein degraders. A total of US$5.7bn is on the table for the new development of an anti-inflammatory candidate that is not part of Monte Rosa’s pipeline.
The FDA under President Trump is heading for a radical break with decades of practice: the external advisory committees that have long served as critical checks in approval decisions are to be largely sidelined. George Tidmarsh, head of the Centre for Drug Evaluation and Research, has dismissed the panels as redundant and instead wants to rely on the publication of Complete Response Letters (CRLs). Officially, the move is framed as efficiency – in reality, it raises serious questions about the agency’s transparency.
The Association of the British Pharmaceutical Industry (ABPI) has warned that the UK could miss its target of becoming the number one in European life sciences by 2030.
CAR-T cell therapy has demonstrated remarkable success in treating hematologic malignancies and is now expanding into solid tumors. On June 1, 2025, The Lancet published positive results from the world’s first randomized controlled trial of CLDN18.2 specific CAR-T in gastric cancer, led by Professor Lin Shen’s team at Peking University.
British antibody specialist Alchemab Therapeutics Ltd has secured a US$32m Series A extension after it completing preclinical development of ATLX-1282 and starting a Phase I clinical trial.


 wikipedia - PyMOL rendering of PDB
wikipedia - PyMOL rendering of PDB
 Henk Vrieselaar - stock.adobe.com
Henk Vrieselaar - stock.adobe.com  Adobe stock photos - nadia
Adobe stock photos - nadia adobe stock photos - kemol
adobe stock photos - kemol Rosa Therapeutics Inc
Rosa Therapeutics Inc FDA
FDA adobe stock photo - Toko
adobe stock photo - Toko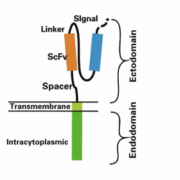
 Alchemab Therapeutics Ltd
Alchemab Therapeutics Ltd