In February 2025, the Federal Office of Public Health (FOPH) announced that the Masterplan in Biomedicine will be discontinued. This sends a troubling signal at a time of growing protectionism and potential trade conflicts. While other nations are actively collaborating with industry to secure the future of their pharmaceutical sectors, Switzerland is abandoning the only federal initiative aimed at improving conditions for biomedical research. The organisation scienceindustries calls for a renewed effort by the government to develop a new master plan.
ADVERTISEMENT
BioSpring GmbH has broken found for one of the world’s largest production facilities for pharmaceutical nucleic acid-based therapeutics. The new high-tech plant will significantly expand the company’s manufacturing capacity for RNA- and DNA-based active pharmaceutical ingredients, and will support Good Manufacturing Practice (GMP)-compliant production for large-scale projects on behalf of BioSpring’s global partners.
The case of the psoriasis antiinflammation antibody guselkumab is a story of deals and cooperation and selling and buying royalties to fuel other innovation paths. J&J is right in the middle of the game and has now full ownership while expanding the indications further.
NovoArc GmbH, an Austrian biotech startup and BIOTECH AUSTRIA member, develops lipid-based formulations to enable the oral delivery of drugs that currently require injection. Its innovative approach paves the way for more convenient and effective treatments.
Swiss MoonLake Immunotherapeutics AG has secured up to US$500m in non-dilutive financing from life sciences debt specialist Hercules Capital to finance the potential 2027 launch of sonelokimab in hidradenitis suppurativa and clinical trials of the IL-17 dimerisation blocker in palmoplantar pustulosis and other inflammatory indications.
Biotechnology provides the necessary toolbox, ranging from energy-saving biocatalysts for the chemical industry to climate-friendly production of animal feed and food proteins for more resilience, microbial recovery of rare critical materials, to the production of pharmaceutically active ingredients.
3PBIOVIAN, a leading Contract Development and Manufacturing Organization (CDMO) with locations in Pamplona (Spain) and Turku (Finland), has been awarded the prestigious CDMO Leadership Award 2025 in the “Cell & Gene Therapy International” category. This award recognizes the excellence of the services that 3PBIOVIAN provides to the Advanced Therapies industry, solidifying its position as a leader in the international sector.
Therapeutic oligonucleotides are rapidly transforming drug development by enabling precise gene modulation strategies. Microsynth empowers biotech, pharmaceutical, and academic researchers with high-quality therapeutic oligonucleotides, supporting every stage from sequence design to preclinical development. With tailored solutions, advanced automation, and expert design support, we accelerate your therapeutic programmes with precision and speed.
Europe’s decision on how to combine economic growth and decarbonisation in the future depends on the renewal of the EU bioeconomy strategy. The question is whether the EU will fall further behind globally or whether bioeconomy will become the EU’s growth engine.
Freiburg-based Eleva relies on its moss-based platform for the development of new biologics both independently and with partners. A new alliance with Spanish CDMO 3PBIOVIAN now secures clinical production capacity for both activities.


 Freepik.com
Freepik.com BioSpring
BioSpring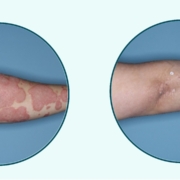 Johnson & Johnson
Johnson & Johnson NovoArc
NovoArc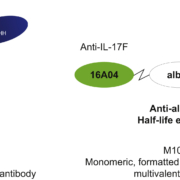 American Academy of Dermatology;doi.org/10.1016/j.jaad.2019.03.056
American Academy of Dermatology;doi.org/10.1016/j.jaad.2019.03.056 BRAIN Biotech AG
BRAIN Biotech AG
 Microsynth AG
Microsynth AG Audiovisual Librairy of the European Commission
Audiovisual Librairy of the European Commission Eleva GmbH
Eleva GmbH