As the regulatory strategy becomes a boardroom-level concern for biotech executives pursuing global expansion, knowing how the Saudi Food and Drug Authority (SFDA) and the European Medicines Agency (EMA) differ is crucial, particularly as Saudi Arabia accelerates its ambition to become a global life sciences hub. Although both regulators adhere to international norms, their differences in review speed, procedural flexibility, and strategic positioning can have a direct impact on competitive advantage, investment planning, and time to market.
ADVERTISEMENT
Tag Archive for: EMA
Belgian lysosomal storage disease specialist Azafaros BV has added a new lead investor to its portfolio. In addition to €25m Series A leader Forbion, Jeito Captial co-led a €132m Series B round aimed at advancing two pivotal trials of Azafaros‘ lead compound nizabagloustat (AZ-3102).
The EMA has asked the industry to share their thoughts on its draft guidelines for developing and assessing biosimilars. The idea is to make it easier for patients in the EU to get access to biosimilars, while also keeping Europe a good choice for developers.
The Safety Committee PRAC of the European Medicines Agency EMA is investigating a new potential adverse reaction to the appetite suppressant semaglutide. Epidemiological studies indicate that the rare eye disease non-arteritic anterior ischaemic optic neuropathy (NAION), which leads to blindness, could occur more frequently after treatment with the GLP-1 receptor agonist.
As the first Nordic company seeking Novel Food approval for a mycoprotein ingredient, Finnish Enifer has filed a authorisation application for it brand PEKILO to the EU food watchdog EFSA.
Danish vaccine maker Bavarian Nordic A/S has submitted data to the EMA that support label extension of its mpox vaccine imvanex indication to include adolescents 12 to 17 years of age.
The European Commission has fully upheld the complaint by PharmaMar SA due to conflicts of interest of leading EMA experts in the (non-)authorisation of Aplidin.
EMA’s human medicines committee (CHMP) recommended ten medicines for approval at its June 2024 meeting.
Born with the aim of harmonising the application, evaluation, and supervision process of clinical trials conducted in EU countries, there is much room for improvement for the Clinical Trial Information System CTIS, particularly with regard to SMEs and translational researchers.
In 2016, PharmaMar filed a European marketing authorisation application for Aplidin as a treatment for patients with multiple myeloma. After the European Medicines Agency rejected the MAA twice, which was accepted in Australia with the same data, PharmaMar sued the EC. European Biotechnology spoke with PharmaMar’s Chairman José María Fernández Sousa-Faro.



 Romi - adobe stock.com
Romi - adobe stock.com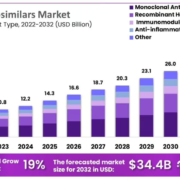 market.us
market.us adobe stock.com - HomuGallery
adobe stock.com - HomuGallery  Enifer
Enifer Bavarian Nordic A/S
Bavarian Nordic A/S


