Swedish biotech Cereno Scientific today announced the expansion of its clinical development program for its investigational small-molecule therapy, CS014, targeting rare lung diseases.
ADVERTISEMENT
Tag Archive for: Clinical trials
The European Commission has unveiled the first part of its draft for a Biotech Act, focusing on health and medical biotechnology. The proposal has won strong support from industry stakeholders, who say it addresses the urgent need for faster innovation processes to retain and develop biotech in Europe.
Spain has once again secured the top position in Europe for clinical trials. According to various Clinical Research Organisations at BioSpain 2025 (7–9 October, Barcelona), this is largely due to the speed with which the Spanish Medicines Agency (AEMPS) approves clinical studies.
The Association of the British Pharmaceutical Industry (ABPI) has warned that the UK could miss its target of becoming the number one in European life sciences by 2030.
Norway-based Calluna Pharma A/S has initiated patient recruitment in late August for its Phase II AURORA trial investigating safety and efficacy of CAL101, a monoclonal antibody with unique mode of action to cure idiopathic pulmonary fibrosis.
Novartis AG has reported a clinical milestone in the treatment of Sjögren’s syndrome, with its BAFF receptor blocker Ianalumab (VAY736) becoming the first investigational therapy to demonstrate a statistically significant reduction in disease activity in pivotal Phase III trials, as measured by the ESSDAI score.
UK focuses on digital health research: £600m (US$764m) for new data platform according to the five recommendations of the Sudlow report.
The Paul Ehrlich Institute (PEI) has approved expansion of the global INVISE Phase 1 clinical trial to Europe. The study aims to evaluate first-in-class gene therapy for the treatment of B-cell malignancies.
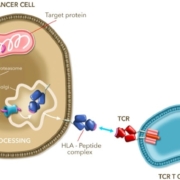
After meeting the primary endpoints of a pivotal Phase II trial, UK-based Adaptimmune Ltd. is submitting an FDA Biologics License Application for its autologous T-cell receptor therapy (TCR-T) Lete-cel (letetresgene autoleucel).
Over the past few years, the Frankfurt Institute of Clinical Cancer Research (IKF) has successfully conducted over 90 clinical studies. Each year, it enrolls approximately 1,500 patients in these trials. European Biotechnology spoke with the founder and director Prof. Dr med. Salah-Eddin Al-Batran.



 EC- press service
EC- press service AseBio
AseBio adobe stock photo - Toko
adobe stock photo - Toko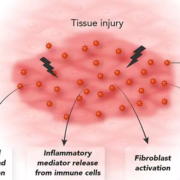 Calluna Pharma A/S
Calluna Pharma A/S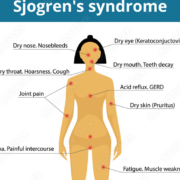 lkov - adobe stock photo
lkov - adobe stock photo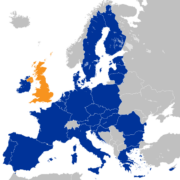 wikicommons
wikicommons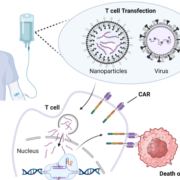
 Adaptimmune
Adaptimmune