Roche inks US$2bn biobucks deal with Sangamo
After Sangamo Therapeutics Inc. presented an epigenetic ZFN-based repressor at ASGCT 2024 that reduces the expression of the tau protein in the hippocampus by 95% in Alzheimer's models, Roche's US subsidiary Genentech has licensed the experimental gene therapy.
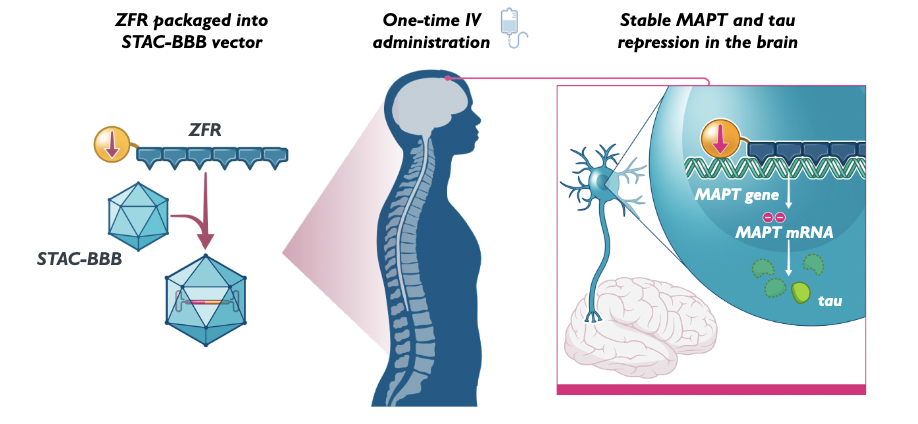
The deal worth US$2bn biobucks includes a zink-finger-protein-based repressor blocking MAPT (Microtubule Associated Protein Tau (MAPT) exon 1 and thus tau expression to treat Alzheimer’s and other tauopathies and the intravenously administered AAV vector STAC-BBB assuring safe capsid-based delivery of the gene fragment across the blood-brain-barrier to the hippocampus. Under the deal that includes application of Sangamo’s epigenetic regulator plus delivery in nearly a dozen tauopathies, Sangamo is set to receive US$50m upfront plus near-term upfront license fees and milestone payments and is eligible to earn up to $1.9 billion in development and commercial milestone payments across multiple medicines, as well as tiered royalties on net sales.
According to Sangamo Therapeutics group leader Amy Pooler, Sangamo’s zinc finger repressor lead, which had been screened out of 384 candidates, blocked transcription of human MAPT by more than 95% with no off-target effects on single-cell level and after CNS delivery in p-tau and htau mice. In all models, the ZFR treatment showed no dose-limiting toxicity. Sangamo has also demonstrated widespread central nervous system (CNS) ZFR expression and MAPT
knockdown in adult nonhuman primates (NHPs) following a single IV administration of
STAC-BBB. An IND-enabling GLP toxicology study for the STAC-BBB-delivered ZFR for the potential treatment of tauopathies, such as Alzheimers or Progressive Supranuclear Palsy, is underway.
The license agreement of Genentech with Sangamo follows the recent opening of a €90m gene therapy centre at Roche’s European Penzberg site, which will focus on IV-administered AAV-based gene therapies set to cure neurological and metabolic and blood disorders. The deal includes a second, not disclosed neurological target and Sangamo’s proprietary, neurotropic adeno-associated virus (AAV) capsid, STAC-BBB.
“The recent discovery of our industry-leading intravenously delivered AAV capsid, STAC-BBB, has the potential to address longstanding challenges in delivering therapeutics to the central nervous system,” said Sandy Macrae, Chief Executive Officer of Sangamo. “We strongly believe in the power of our zinc finger technology to regulate the expression of key genes involved in disease.
According to the contract, Sangamo will be responsible for completing a technology transfer and certain preclinical activities, and Genentech will carry out all clinical studies, regulatory interactions, manufacturing and global commercialisation.


 Araris Biotech AG
Araris Biotech AG
