Spanish Tetraneuron secures financing to advance its E2F4DN-based Alzheimer’s gene therapy, restoring neuronal homeostasis and halting disease progression in Europe.
ADVERTISEMENT
Tag Archive for: CGT
UK-bad Cellular Origins Ltd raised $40m to scale global CGT manufacturing with robotic automation, boosting access to cell therapies and enabling commercial growth.
British gene and cell therapy manufacturing specialist Cellular Origins Ltd has entered into a collaboration with Johnson & Johnson to develop a scalable, end-to-end automated platform for autologous CAR-T cell manufacturing.
After Sangamo Therapeutics Inc. presented an epigenetic ZFN-based repressor at ASGCT 2024 that reduces the expression of the tau protein in the hippocampus by 95% in Alzheimer’s models, Roche’s US subsidiary Genentech has licensed the experimental gene therapy.
The US Food & Drug Administration has granted accelerated approval to Adaptimmune’s Tecelra as the world’s first therapy with genetically engineered T cells to fight a solid tumour.
Evotec SE is widening its 2022 R&D collaboration SynaptixBio Ltdi to optimise and identify new antisense oligonucleotides to treat children with H-ABC leukodystrophy, a rare genetic myelin synthesis defect that shows similar symptoms such as multiple sclerosis.
Basel-headquartered Roche AG has inaugurated a brand new €90m gene therapy development centre in Penzberg near Munich.
Bayer SE subsidiary Asklepios BioPharmaceutical Inc. (AskBio) has started Phase II testing of AB-1002 (NAN-101) in patients with congestive heart failure.
Biovian and 3P Biopharmaceuticals have created a new pan-European CDMO, dubbed 3PBIOVIAN.
British and Italian researchers have demostrated promising results in patients with progressive multiple sclerosis through stem cell transplantation into the brain.


 Tetraneuron SL - Álvaro García
Tetraneuron SL - Álvaro García Cellular Origins Ltd
Cellular Origins Ltd Cytiva/Cellular Origins
Cytiva/Cellular Origins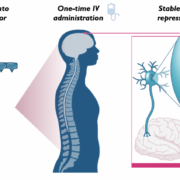 Sangamo Therapeutics Inc
Sangamo Therapeutics Inc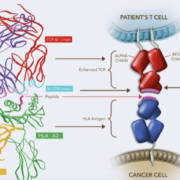 Adaütimmune Ltd
Adaütimmune Ltd Evotec SE
Evotec SE