CRISPR screen highlights genes behind T cell plasticity in autoimmunity
Researchers at University Hospital Hamburg-Eppendorf have laid the foundation to screen for molecules that shift Th17 cells from proinflammatory or immune-dampening mode.
Pro-inflammatory CD4-positive T cells are major drivers of autoimmune diseases, yet therapies modulating T cell phenotypes to promote an anti-inflammatory state are lacking. Modulating plasticity, the ability of T cells to adopt an pro-inflammatory or an immune-dampening state, in pathogenic T cells to promote a tolerogenic phenotype is a promising therapeutic approach in autoimmunity and cancer. To explore how pathogenic TH17 cell plasticity is regulated in mouse models of kidney and gut inflammation, Leon Enk and colleages at University Hospital Hamburg-Eppendorf optimised an in vivo pooled single-cell CRISPR droplet sequencing (iCROP-seq) screen that allowed the assessment and quantification of CRISPR-modified TH17 cell polarisation states in vivo.
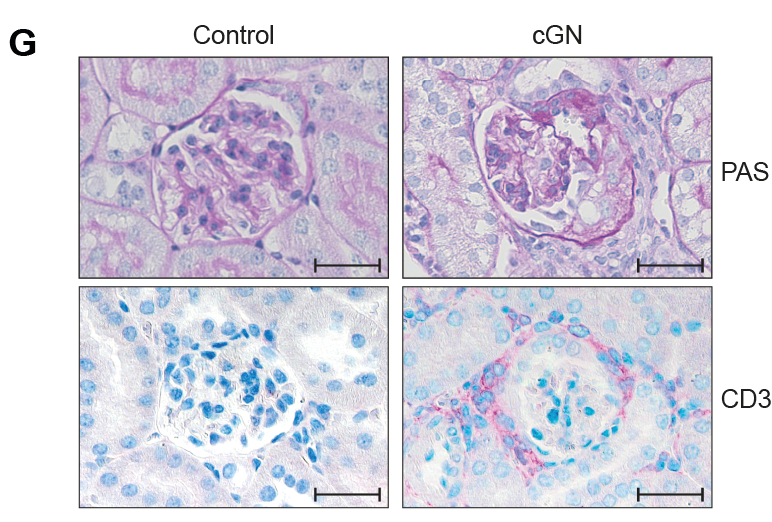
CRISPR-based gene targeting in TH17 cells could be ranked according to the resulting transcriptional perturbations, and polarisation biases into T helper 1 (TH1) and regulatory T cells could be quantified. Furthermore, the researchers demonstrated that iCROP-seq can facilitate the identification of therapeutic targets by efficient functional stratification of genes and pathways in a disease- and tissue-specific manner. These findings uncovered TH17 to TH1 cell plasticity in the human kidney in the context of renal autoimmunity.
Thus, using iCROP-seq helped Enk et al. to identify the genes behind Th17 cell plasticity in mouse kidney and gut inflammation models. The researchers also noted this plasticity in kidney biopsies of 11 patients with the autoimmune disease antineutrophil cytoplasmic antibody (ANCA)–associated glomerulonephritis based on single-cell (sc) T cell receptor analysis and scRNA velocity The findings open an avenue to uncover molecules that drive T cells to adopt either pro-or anti-inflammatory states.
With further study, clinicians may be able to manipulate this plasticity therapeutically in ANCA-glomerulonephritis and other auto-immune diseases such as inflammatory bowel disease (IBD) or colitis ulcerosa. “Allowing the screening of numerous therapeutic candidates in one experiment, iCROP-seq is a powerful tool to understand disease mechanisms at the molecular level,” said group leader Christian Krebs. “This could accelerate translational research and help to bridge the translational gap between the identification of transcriptional differences and clinical application by preselecting the most promising candidates for functional testing.”


 VedaBio Inc
VedaBio Inc
 Bayer AG
Bayer AG