Alvotech Advances in Biosimilar Market with Successful AVT03 Study
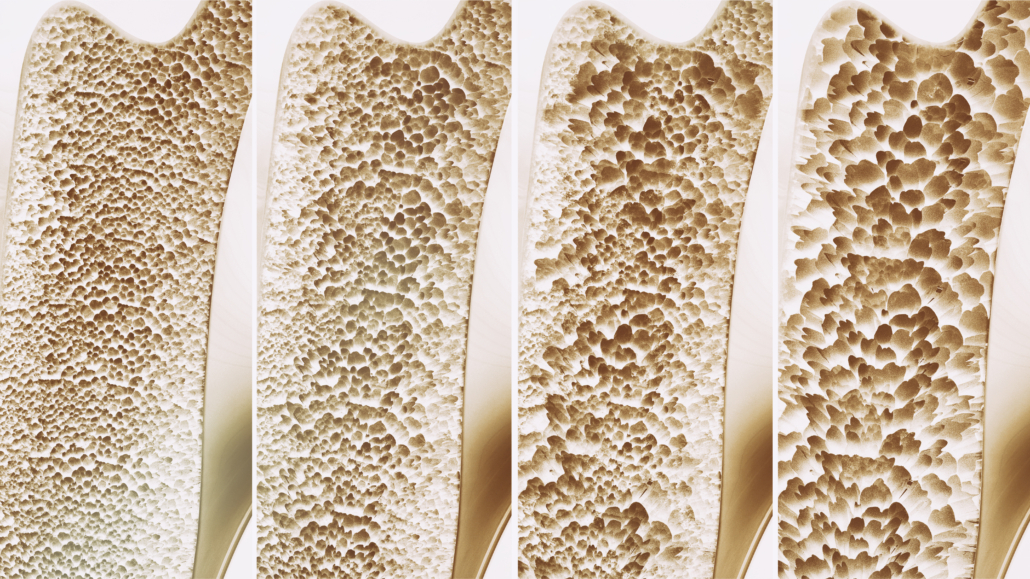
AVT03, a biosimilar candidate for osteoporosis treatment, showed promise in treating bone illnesses, according to results from a pharmacokinetic study by Alvotech SA.
Alvotech SA, a global biotech company from Luxembourg, specialising in biosimilars, reported positive results from a pharmacokinetic study of AVT03, a proposed biosimilar for Prolia® and Xgeva®, both containing denosumab, a human monoclonal antibody for the treatment of osteoporosis and preventing skeletal-related events in cancer. The combined sales of these drugs exceeded US $ 6 bn in the year before September 30, 2023. The study, which met its primary endpoints, assessed AVT03’s pharmacokinetics, safety, and tolerability compared to Prolia® in healthy adults. Further studies on AVT03 are ongoing.
Alvotech focuses on developing and manufacturing biosimilars, aiming to be a global leader in this field. The company’s pipeline includes biosimilars for various medical conditions, including autoimmune disorders, eye and respiratory diseases, osteoporosis, and cancer. Chief Scientific Officer Joseph McClellan highlighted Alvotech’s proficiency in biosimilar development. AVT03, a human monoclonal antibody, is yet to receive regulatory approval and is still under investigation. It targets the RANK ligand to reduce bone resorption and cancer-induced bone destruction.



 EC- press service
EC- press service AseBio
AseBio