Italian researchers have successfully ameliorated several IL-1-based autoimmune disorders by gene therapy into transplantable hematopoietic stem cells of mice.
ADVERTISEMENT
Physiologically based pharmacokinetic (PBPK) modelling is a tool that can have a huge impact in speeding up drug development. It simulates a range of physiological factors that have an effect on how an orally dosed drug behaves, flagging up any areas where performance might be expected to be sub-optimal. This allows them to be addressed at an early stage, rather than causing bigger problems later on that might hold up development while a solution is sought.
Real Quality. Real Results. With cell & gene therapies, the process is the product – so ensuring high-quality starting material is a critical first step in maximizing your results.
Bristol-based eXmoor Pharma Ltd has completed a US$35m Series A financing to expand its cell and gene therapy manufacturing capabilities.
Leiden-based VarmX BV said it will use the €30m Series B2 money to complete the data required for IND approval of its lead candidate VMX-C001, a recombinante antidote to oral factor Xa inhibitors which are widely used to prevent blood clotting but can lead to dangerous bleeding episodes. The proceeds will also enable VarmX to scale manufacturing of VMX-C001 for its further clinical testing. The financing was led by Sound Bioventures the EIC Fund joining existing investors EQT Life Sciences (formerly LSP), Inkef, Lundbeckfonden BioCapital, Ysios Capital, BioGeneration Ventures, InnovationQuarter, and Libertatis Ergo Holding.The Dutch company had already completed a €32m Series B financing in 2020.
VarmX has completed enrollment of patients into a first-in-human study, to demonstrate safety and provide clinical proof of principle of the compound. The company will present initial data at the International Society on Thrombosis and Haemostasis conference in Montreal (June 24-28, 2023). Full study results of the study that is set to enroll 88 patients will be available later this year.
VMX-C001 is a modified recombinant human blood clotting factor X derived from the Australian brown snake venome that enables blood to clot normally in the presence of synthetic factor Xa blood clotting inhibitors. About 2-3% of the annually 10 million patients that receive Factor Xa blood clotting blockers suffer from severe bleeds. VMX-C001 is set to facilitate the fast, safe and effective treatment of severe spontaneous bleeds in patients that are taking direct oral anticoagulant blood thinners (DOACs) and, in addtion, to enable patients on this class of blood thinners to undergo emergency surgery without the risk of bleeding associated with FXa DOACs.
The compound has the potential for a strong and differentiated profile, including universal and single dose reversal, ease-of-use and safety that supports emergency care use and it shows promise for applications in other indications.
For a long time, scientists have been searching for enzymes that can degrade commodity plastics such as Polyurethanes or poly-vinyl chloride (PVC). German-US company Covestro AG and researchers at University of Greifswald now say that they have found new enzymes that could do the job.
German Bayer AG opens first Co.Lab life science incubator in Cambridge/Boston. This comprises state-of-the-art laboratories and collaborative workspaces specifically designed to support entrepreneurs focused on developing cell and gene therapies.
Transmembrane proteins play a variety of roles in physiological processes such as protein ligation, recognition, transport, anchoring, and transduction.
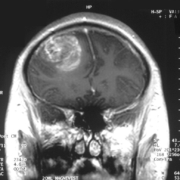
An anti-TNF-antibody-cytokine fusion combo with chemotherapy turns inoperable brain tumours hot in mice. A Phase I trial sponsored by Philogen SpA is underway.
The BioRN Life Science cluster Rhine-Neckar has set the right course to further accelerate the start-up dynamic. Several lighthouse projects have been established recently, fostering innovation and providing the ideal breeding ground for biotech projects. Joint forces now develop strategies to attract investors and increase the visibility of the start-up community. The Life Science Investors´ Day Heidelberg has established itself as a top-class platform.