So far, no gene and cell therapies have been approved to treat solid tumours. European Biotechnology spoke with Karen Miller, SVP Pipeline Research at Adaptimmune plc, about promising candidates, and the art of circumventing supply chain bottlenecks caused by the COVID-19 pandemic.
ADVERTISEMENT
Richter-Helm, a leading steadily growing Germany-based contract development and manufacturing organization (CDMO), offers excellent services in the continuously growing market of pharmaceutical biotechnology. Two additional bioreactors with capacities of 300 litres and 1,500 litres for microbial production will be added. This includes also the establishment of mid- and downstream processing and supporting utilities on a total area of about 10,000 m².
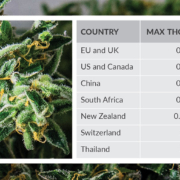
For a plant that has been used for millennia, controls on the supply of psychoactive and non-psychoactive components extracted from cannabis are relatively recent, with global regulations starting in the 20th century with the International Opium Conventions. The current, and often conflicting, regulatory landscape has focused on definitions of hemp and cannabidiol (CBD). Reference materials are critical to defining the % ?9-THC in these products.
Ultra-low freezers, or ULTs, are specialised refrige-rating equipment created to store vaccines, medicines, and samples at ultra-low temperatures. By offering storage temperatures ranging from -20°C to -86°C and energy efficiency, B Medical Systems’ ULTs create an ideal environment for the long-term storage of extremely temperature-sensitive biologicals, making its products perfect for use in laboratory or clinical environments.
Lonza will expand its production capacity of Moderna’s mRNA vaccine mRNA1273 at its manufacturing site in Geelen, the Netherlands.
The European Medicines Agency (EMA) has given the green light for vaccination of adolescents with Biontech’s COVID-19 vaccine Comirnaty (BNT162b2).