In biomedical research and biomanufacturing, the purity of reagents directly impacts the reliability of experimental outcomes and the safety of downstream therapeutic products. Endotoxins are notorious contaminants in protein preparations. Even trace amounts of endotoxin in recombinant proteins can trigger potent immune responses, distort results, and compromise patient safety, especially in sensitive applications such as immunology, cell and gene therapy, and vaccine production. Sino Biological has addressed this urgent need with the launch of ProPure™, an advanced line of endotoxin-free recombinant proteins.
ADVERTISEMENT
Tag Archive for: sino biological
CAR-T cell therapy has demonstrated remarkable success in treating hematologic malignancies and is now expanding into solid tumors. On June 1, 2025, The Lancet published positive results from the world’s first randomized controlled trial of CLDN18.2 specific CAR-T in gastric cancer, led by Professor Lin Shen’s team at Peking University.
Stick, Tag, Destroy: The Science of PROTACs and MGDs – For a long time, traditional drug research focused on inhibitors that block the active sites of disease-related proteins. However, around 80% of these proteins remain “undruggable” because they lack clear binding sites.
Antibody-Drug Conjugates (ADCs) have been transformative in oncology, offering targeted cancer treatments that enhance efficacy while sparing healthy cells. However, the potential of ADCs extends well beyond cancer care. From autoimmune diseases to metabolic disorders, advancements in ADC technology are paving the way for breakthroughs across a range of therapeutic areas. This article explores how ADCs are redefining treatment paradigms and improving patient outcomes in diverse fields of medicine.
High-Quality HIV Antigens for Cutting-Edge Solutions
Neurodegenerative diseases like Alzheimer’s disease (AD), Parkinson’s disease (PD), and amyotrophic lateral sclerosis (ALS) suffer from a scarcity of effective treatments. Current therapies primarily provide symptomatic relief but do not address the underlying pathology, failing to slow or halt disease progression. This underscores the urgent need to identify new therapeutic targets relevant to the pathology of these conditions. Kinases represent highly tractable drug targets, with kinase inhibitors achieving significant success, particularly in oncology. However, their potential in neurodegenerative diseases remains largely unexplored, presenting a promising avenue for future research and therapeutic development.
Sino Biological, a leading recombinant protein production company, is pleased to announce an expansion of its strategic partnership with BioGeometry, a pioneer in digital biology. This collaboration brings together Sino Biological’s advanced protein expression and wet-lab capabilities with BioGeometry’s generative AI protein design and optimization platform. The two companies aim to enhance their joint offerings and explore high-value market opportunities globally.
Antibody-drug conjugates (ADCs) are a new class of biotherapeutics, consisting of acytotoxic payload covalently bound to an antibody by a linker. Evaluating the pharmacokinetics (PK) properties of ADCs in preclinical and clinical studies is essential for their strategic design and successful development.
Sino Biological, Inc., is pleased to announce the formal opening of its new Center for Bioprocessing (C4B) in Houston, Texas USA, adjacent to the world-renowned Texas Medical Center.
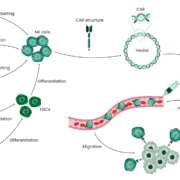
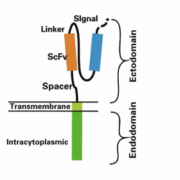
CAR-T therapies represent a new successful strategy for the treatment of hematological malignancies; however, recent studies have revealed some limitations, including the occurrence of graft-versus-host disease (GVHD), neurotoxicity, and cytokine release syndromes (CRS). This has prompted researchers to look for alternative, safer cells, such as natural killer (NK) cells 1. NK cells are lymphocytes that kill tumor cells and virus-infected cells non-specifically, without prior sensitization. CAR-NK therapy involves the modification of NK cells with mature chimeric antigen receptor (CAR) technology to exploit their unique target cell recognition mechanism and broad tumor-killing ability. Figure 1 illustrates the CAR-NK therapeutic strategy 1. Compared with CAR-T therapy, CAR-NK therapy has numerous advantages, such as lower GVHD and significantly reduced CRS toxicity and immune effector cell-associated neurotoxicity syndrome toxicity, which enhance overall safety 2, 3. In addition, NK cells have multiple tumor recognition sites, thus potentially reducing antigenic escape failure 4.



 Sino Biological
Sino Biological Sino Biological
Sino Biological Sino Biological
Sino Biological
 Sino Biological
Sino Biological