The US Roche unit Genentech Inc has signed a US$400 million licence deal with British NativeMS specialist OMass Therapeutics Ltd. Under the exclusive collaboration and licence agreement, Genentech intends to commercialise therapies for inflammatory bowel disease.
ADVERTISEMENT
Tag Archive for: Roche
Pilatus Biosciences AG, a Swiss biotechnology company headquartered in Epalinges with operations in the United States and South Korea, has entered into a clinical trial collaboration with Roche AG. As part of the agreement, Roche will provide its PD-L1 immune checkpoint inhibitor atezolizumab for use in a Phase I study evaluating the combination of atezolizumab with Pilatus’s investigational CD36 inhibitor, PLT012, in patients with hepatocellular carcinoma (HCC).
Immunotherapy company Centauri Therapeutics Ltd, based in Alderley Park, Cheshire, UK, has announced the appointment of Dr Debra Barker as Chief Medical Officer (CMO).
German researchers have identified a promising new target to stop the inflammatory response in ulcerative colitis—a breakthrough that could help prevent the progression to colorectal cancer. Phase II clinical trials of a corresponding monoclonal antibody, licensed by Roche, are expected to begin soon.
For nearly two decades, Roche AG has been searching for a next-generation sequencing method in personalised medicine to overcome the limitations of short read lengths in existing sequencing technologies. Following beta testing, the company plans to launch its new Sequencing by Expansion (SBX) technology commercially next year.
Despite the World Trade Organisation’s (WTO) ban of tariffs on pharmaceuticals, US President Donald Trump has announced he would consider imposing tariffs on pharmaceutical products. In a fast reaction, Swiss pharmaceutical giants Roche and Novartis, which are heavily dependent on the US market, have announced upcoming investments in the US as a production location by 2030.
Roche is extending its obesity pipeline signing a US$5.3bn biobucks deal with Zealand Pharma A/S, who will co-develop and co-commercialise a monotherapy of its long-acting amylin analogue petrelintide with the Swiss pharma giant in the US and Europe and do the same for a combo of petrelintide and Roche’s GLP1/GIP receptor agonist CTT388.
Roche researchers have introduced a greatly improved long-read nanopore sequencing method that has the potential to erase the sequencing-by-synthesis approach of market leader Illumina.
Swiss Noema Pharma AG has cashed in a CHF27m extention to its Series B financing that is used to advance four Phase II programmes in neurology.
Genentech, the US arm of Swiss-based Roche AG, plans to acquire its long-time partner in allogeneic CAR-T blood cancer and gene therapies, Poseida Therapeutics Inc, for up to US$1.5bn.


 adobe stock photos - Sundry Photography
adobe stock photos - Sundry Photography 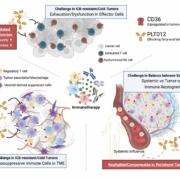 Pilatus Biosciences
Pilatus Biosciences Centauri Therapeutics
Centauri Therapeutics MichaelVi - stock.adobe.com
MichaelVi - stock.adobe.com Adobe Stock - TechArtTrends
Adobe Stock - TechArtTrends  adobe stock - peterschreiber.media
adobe stock - peterschreiber.media  asobe stock photos - catalin
asobe stock photos - catalin  Roche AG
Roche AG Pixabay- Deltaworks
Pixabay- Deltaworks Roche, Herzog&De Meuron
Roche, Herzog&De Meuron