British cell and gene therapy CDMO eXmoor Pharma plc, and KU Leuven which has just launched a translational cell and gene therapy hub in Belgium have inked a strategic collaboration supporting the hub’s work in four areas.
ADVERTISEMENT
Tag Archive for: CDMO
Just three weeks to go until Europe’s largest event for laboratory automation and drug screening. SLAS Europe 2025, organised by the Society for Laboratory Automation and Screening (SLAS), will take place from 20-22 May in Hamburg, Germany. In a series, European Biotechnology presents the 12 most innovative SMEs. Today: Nanoworx BV.
French ERBC and Menarini Biotech from Italy forge a new strategic alliance to accelerate biopharmaceutical development.
BioSpring GmbH has broken found for one of the world’s largest production facilities for pharmaceutical nucleic acid-based therapeutics. The new high-tech plant will significantly expand the company’s manufacturing capacity for RNA- and DNA-based active pharmaceutical ingredients, and will support Good Manufacturing Practice (GMP)-compliant production for large-scale projects on behalf of BioSpring’s global partners.
3PBIOVIAN, a leading Contract Development and Manufacturing Organization (CDMO) with locations in Pamplona (Spain) and Turku (Finland), has been awarded the prestigious CDMO Leadership Award 2025 in the “Cell & Gene Therapy International” category. This award recognizes the excellence of the services that 3PBIOVIAN provides to the Advanced Therapies industry, solidifying its position as a leader in the international sector.
German API and antibody drug conjugate (ADC) CDMO Axplora Group GmbH has announced an investment of €50m in its Mourenx site in Southwest France, securing its position as a key player in the fight against cardiovascular and metabolic diseases.
The Strasbourg-based biotech company Transgene and the Berlin-based CDMO ProBioGen have entered into a licensing agreement to collaboratively advance the development of individualised cancer vaccines.
Rentschler Biopharma announces largest single investment in the higher double-digit range at its headquarters in Germany for construction of a new buffer media facility in Laupheim.
Biomanufacturers must balance the development of new therapeutic modalities and reduce manufacturing costs while maintaining environmental responsibility. Efforts are centred on improving process robustness and efficiency and creating more flexible manufacturing facilities that can accommodate diverse biopharmaceutical products.
ARTES offers a brand-new business area making the hearts of all R&D staff beat faster. We have a whole range of first-class antigens, enzymes and functional proteins just waiting to be used in new, exciting projects.


 adobe stock - aliaksandr
adobe stock - aliaksandr Nanoworx BV
Nanoworx BV Menarini Group
Menarini Group BioSpring
BioSpring
 Axplora Group
Axplora Group ProBioGen
ProBioGen Rentschler Biopharma SE
Rentschler Biopharma SE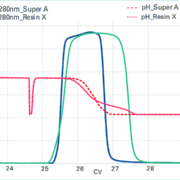 Tosoh Bioscience
Tosoh Bioscience