Amsterdam-based UniQure NV’s stocks made a 240% after-hours jump at Nasdaq after the company announced its Huntington gene therapy AMT-130 met the endpoints of a Phase II study and it will apply for marketing authorisation next year. In addition, UniQure entered into a US$175m non-dilutive senior secured term loan facility with Hercules Capital and commenced a US$200m underwritten public offering of its ordinary shares to prepare a BLA in Q1 2026.
ADVERTISEMENT
Tag Archive for: BLA
After meeting the primary endpoints of a pivotal Phase II trial, UK-based Adaptimmune Ltd. is submitting an FDA Biologics License Application for its autologous T-cell receptor therapy (TCR-T) Lete-cel (letetresgene autoleucel).


 uniqure
uniqure 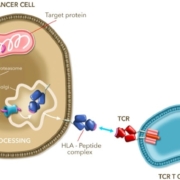 Adaptimmune
Adaptimmune