Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X) is an industry alliance to support research and development projects in AMR. Since its inception in 2016, CARB-X has supported 114 R&D projects across 14 countries, leading to 18 projects advancing into or completing clinical trials. EBM talked to CARB-X Executive Director Kevin Outterson.
ADVERTISEMENT
Tag Archive for: AMR
Antimicrobial resistance (AMR) poses a huge threat to the prevention and treatment of a growing number of infections caused by bacteria, parasites, viruses, and fungi. A public-private partnership involving nine European projects and 98 organisations is now calling for long-term investment to maintain Europe’s ability to research and develop new antibiotics.
Swedish antimicrobial specialist Amferia AB has inked a contract is with the Swiss animal health company Biokema SA for commercialisation of Amferia’s proprietary antimicrobial wound care technology in Switzerland.
A Dutch-German resarch team together with Evotec SE have presented a new class of vancomycin derivatives with good safety profile that kill multi-resistant, Gram-positive bugs.
Swiss multivalent vaccine developer LimmaTech Biologics AG has been awarded US$2.2m rom the Combating Antibiotic-Resistant Bacteria Biopharmaceutical Accelerator (CARB-X).
Novo Nordisk Foundation has partnered with CARB-X investing US$25m to help SMEs to fight drug-resistant infections.
Hoffmann-La Roche AG has published details on the mechanism of action of its Phase I antibiotic Zosurabalpin (RG6006) that kills the carbapenem-resistant bug Acinetobacter baumannii.
.
Antibiotics specialist Antabio SAS has raised €25m in a Series B financing round with subscriptions from the AMR Action Fund, the EIC Fund and from existing investors.
In an effort to address the global health threat of antimicrobial resistance (AMR), Innovate UK, LifeArc, and Medicines Discovery Catapult have jointly launched the PACE (Pathways to Antimicrobial Clinical Efficacy) initiative. With £30m (around €34,5m) in funding, PACE aims to support early-stage innovations aimed at countering AMR.
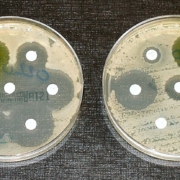
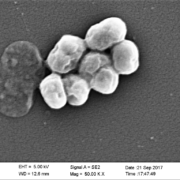
Researchers at the Universities of Bonn (Germany) and Utrecht (The Netherlands and from NovoBiotic Pharmaceuticals (Cambridge, US) have discovered a new class of antibiotics. They isolated clovibactin from the thought-to-be uncultured soil bacterium Eleftheria terrae, subspecies Carolin. It efficiently kills drug-resistant Gram-positive bacterial pathogens without detectable resistance.
Using biochemical assays, solid-state nuclear magnetic resonance, and atomic force microscopy, the team dissect its mode of action. Clovibactin blocks cell wall synthesis by targeting pyrophosphate of multiple essential peptidoglycan precursors (C55PP, lipid II, and lipid IIIWTA). Clovibactin uses an unusual hydrophobic interface to tightly wrap around pyrophosphate but bypasses the variable structural elements of precursors, accounting for the lack of resistance. Selective and efficient target binding is achieved by the sequestration of precursors into supramolecular fibrils that only form on bacterial membranes that contain lipid-anchored pyrophosphate groups. This potent antibiotic holds the promise of enabling the design of improved therapeutics that kill bacterial pathogens without resistance development.
The discovery of clovibactin was made possible by a new technology called the iCHip apparatus. This makes it possible to grow bacteria in the laboratory that were previously considered unculturable.. The mechanism of action of the new antibiotic was elucidated by researchers led by Tanja Schneider of the Institute of Pharmaceutical Microbiology at the University Hospital Bonn. They showed that Clovibactin blocks the synthesis of the bacterial cell wall by binding specifically and with high specificity to pyrophosphate groups on cell wall building blocks (peptidoglycan precursors).
After docking with the target structures, Clovibactin forms supramolecular fiber-like structures that tghtly enclose the pyrophosphate groups – like a cage. Hence the name of the new antibiotic, which is derived from the Greek word "Klouvi" (cage). Bacteria that encounter Clovibactin are also stimulated to release autolysins, which uncontrollably dissolve their own cell wall.
Clovibactin acts primarily on gram-positive bacteria. These include methicillin-resistant Staphylococcus aureus (MRSA), as well as the pathogens that cause tuberculosis. "We are very confident that the bacteria will not develop resistance to clovibactin as quickly," Schneider says. He adds that the combination of the different mechanisms of action results in "exceptional resistance to resistance."
Moreover, the pyrophosphate groups to which Clovibactin docks are immutable. This leaves the bacterium with few options to escape the antibiotic through mutation. Next, the research team plans to use its findings to date to further increase the effectiveness of Clovibactin. However, Schneider says there is still a long way to go before the new antibiotic reaches the market.
Researchers at Northeastern University have previously used the iChip to find another antibiotic called teixobactin.


 CARB-X
CARB-X Image by freepik
Image by freepik Amiferia AB
Amiferia AB