Elevation Oncology Inc has inked a US$368m license agreement with Lonza subsidiary Synaffix BV under which Synaffix will link Elevation’s HER3 antibody seribantumab to a monomethyl auristatin E (MMAE) toxic payload with broad therapeutic index.
ADVERTISEMENT
Tag Archive for: ADC
Swiss Alentis Therapeutics has raised US$181.4m in an oversubscribed Series D financing set to boost clinical development of its anti-Claudin-1 ADCs in solid tumours.
Monoclonal antibodies still dominate the US$417bn biologics market. After decades of development, however, improved antibodies and protein formats with better target selectivity and safety profiles are now hitting markets and pipelines. A range of bispecific antibodies and antibody-drug conjugates – along with CAR-Ts, TCR-RTs and NK cell-based immunoreceptor constructs – were in focus at the PEGS Europe Summit.
Antibody-drug conjugates (ADCs) are a new class of biotherapeutics, consisting of acytotoxic payload covalently bound to an antibody by a linker. Evaluating the pharmacokinetics (PK) properties of ADCs in preclinical and clinical studies is essential for their strategic design and successful development.
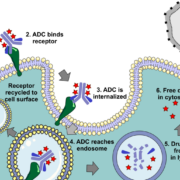
Danish ADC maker Adcendo ApS has licenced the rights to commercialise Multitude Therapeutics Inc’s first-in-class ADCE-T02 globally except the Greater China region.
Good news for BioNTech SE: The safety study of the ADC BNT326/YL202 developed by MediLink Therapeutics, which was suspended by the FDA in mid-June, will continue.
Czech Sotio Biotech has inked an R&D and licence option agreement with Chinese antibody maker Biocytogen.
Tubulis doses first patient in phase 1/2a trial investigating ADC candidate TUB-040 in ovarian cancer and lung adenocarcinoma (NSCLC). TUB-040 is a NaPi2b-targeting Exatecan ADC based on Tubulis’ proprietary linker technology with better biophysical properties that demonstrated effective and durable responses in a range of preclinical models.
Glycan target specialist Tacalyx GmbH expands its seed financing to €14m to advance its anti-TACA cancer therapies.
Genmab announced today that it will acquire privately held biotech company ProfoundBio for $1.8bn in cash. The deal is designed to help the Danish pharmaceutical company deepen its cancer pipeline with next-generation antibody-drug conjugate therapies where the company has already an inlicensing deal with former Synaffix´s ADC technology platform (which was acquired mid of 2023 by Lonza).


 Synaffix/Lanza
Synaffix/Lanza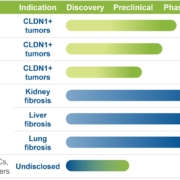 Alentis Therapeutics
Alentis Therapeutics BIOCOM
BIOCOM
 Adcendo ApS
Adcendo ApS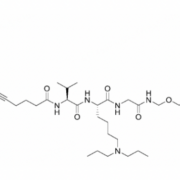 Medilink Therapeutics
Medilink Therapeutics Biocytogen Co Ltd
Biocytogen Co Ltd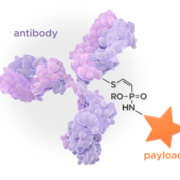 Tubulis GmbH
Tubulis GmbH