Wacker Chemie AG opens €100m RNA manufacturing site
With the opening of a production centRE for RNA vaccines and therapeutics, German CDMO Wacker Biotech is expanding its expertise in the field of RNA active ingredients.
With a new production facility, which Wacker Chemie subsidiary Wacker Biotech calls an RNA competence centre and whose construction costs are estimated at €100m, the contract manufacturer (CDMO) is creating 100 new jobs and building up expertise in the field of RNA vaccines and active ingredients. In April 2022, Wacker, together with contract manufacturer CordenPharma, was awarded a tender by the German government for pandemic preparedness. The groundbreaking ceremony took place on July 1. The RNA competence centre planned and built by Exyte GmbH with four production lines and a total gross floor area of 7,400 m2, including 1,600 m2 as a clean room, has now been officially opened together with 300 invited guests from politics and business.
“In just two years, we have built up a high-tech production facility with an annual capacity of over 200 million vaccine doses,” said Wacker CEO Christian Hartel at the opening ceremony.
Originally, annual investments of around €80m and the recruitment of 200 employees had been planned, at least according to the press release from 2022. The now leaner solution nevertheless makes Wacker an attractive partner when it comes to the production of modern RNA active ingredients for clinical trials and the market.
According to the 5-year contract with the German government, Wacker Biotech and CordenPharma are expected to manufacture 80 million RNA vaccine doses per year to be prepared for the next pandemic. Additionally, the German government has an option to increase the amount to 100 million doses per year. The two companies will receive an annual stand-by fee for keeping production capacity available. The stand-by phase has started in 2024and ends in 2029.
As the potential of RNA meds go far beyond RNA vaccines against corona- or other viruses, the capacity built-up is an indirect subsidy to build manufacturing capacity for RNA cancer vaccines, aptamers, siRNA or RNA-based gene therapies in Germany and thus part of Germany’s pharma strategy that aims to bring the country back to the top pharma and clinical study locations worldwide.
“mRNA technology is bringing enormous progress to medicine, and not just in the field of vaccines. For example, it gives us the opportunity to help people with cancer in a much more targeted way in the future,” explained Melanie Käsmarker, CEO of Wacker Biotech. Wacker Biotech bundles the WACKER Group’s biopharmaceutical activities and, among other things, produces active ingredients for the market and clinical trials in Halle on behalf of pharmaceutical companies. “From Halle, we will serve the growing global demand for mRNA active ingredients in the future,” says Käsmarker.
Wacker’s expansion of its 2014 production centre in Halle is not only dedicated to build production capacities for pandemic vaccines but also to satisfy the demand of other customers in the RNA drug space. Last year, the company together with CordenPharma International GmbH have launched a R&D project with academic researchers from Munich and Berlin to accelerate the development of RNA-based drugs by formulating novel lipid nanoparticles (LNPs), fat droplets used to deliver RNA-based pharmaceuticals at target sites in the body. Based on these formulations, a machine learning algorithm is to be trained that automatically identifies the best constituents for new RNA formulations – as yet a particularly time-consuming and costly development stage. Furthermore contracts with RNA drug developers have already been signed but not specified, according to Wacker.
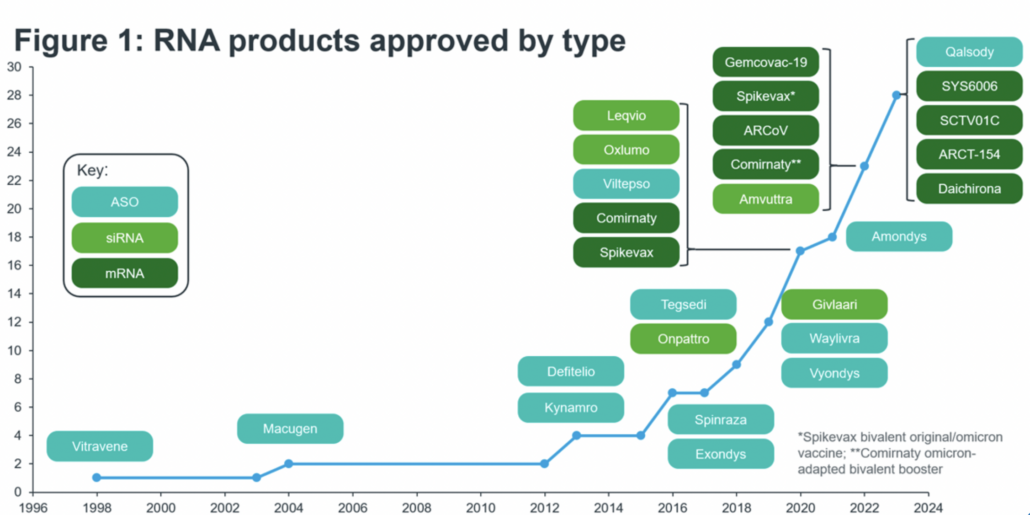
According to an analysis published by IQVIA in November 2023, since the first approval of an antisense oligonucleotide back in 1998 (Vitravene), the RNA market remained small for two decades, with few new product approvals. It has only been since 2016, the year of approval of the US$1.8bn SMA blockbuster Spinraza and – two years later the first siRNA product Onpattro, that a hype in the RNA field emerged peaking in the corona pandemic. As of November 2023 131 RNA products were in clinical development with three quarters being new treatments for neurological (21%), oncological (25%) and cardio-metabolic (32%) diseases.
In the light of an upcoming German gene therapy strategy to be presented on 14 June, Wacker Biotech and CordenPharma seem to be well-prepared to compete with players in other countries, i.e. Lonza in Switzerland that have also built RNA compentence during the pandemic


 Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma Rentschler Biopharma SE
Rentschler Biopharma SE