PRAC: Semaglutide linked to rare eye disease?
The Safety Committee PRAC of the European Medicines Agency EMA is investigating a new potential adverse reaction to the appetite suppressant semaglutide. Epidemiological studies indicate that the rare eye disease non-arteritic anterior ischaemic optic neuropathy (NAION), which leads to blindness, could occur more frequently after treatment with the GLP-1 receptor agonist.
The Pharmacovigilance Risk Assessment Committee (PRAC) of the European Medicines Agency has started a review of medicines containing semaglutide following concerns regarding an increased risk of developing NAION, a rare eye condition, as suggested in two recent observational studies, while two other recent observational studies do not suggest an increased risk.
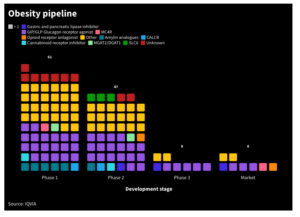
Semaglutide, a GLP-1 receptor agonist, is the Ozempic, Rybelsus and Wegovy) developed by Novo Nordisk, Eli Lilly and others.
PRAC is assessing whether patients treated with semaglutide may have an elevated risk of developing NAION. This is a disorder caused by reduced blood flow to the optic nerve in the eye with potential damage to the nerve, which can lead to loss of vision in the affected eye. Patients with type 2 diabetes might already have an inherently higher risk of developing this condition.
PRAC will now review all available data on NAION with semaglutide including data from
YOU DON`T WANT TO MISS ANYTHING?
Sign up for our newsletter!



 IQVIA
IQVIA