Morphosys’ antibody still shining – for J&J
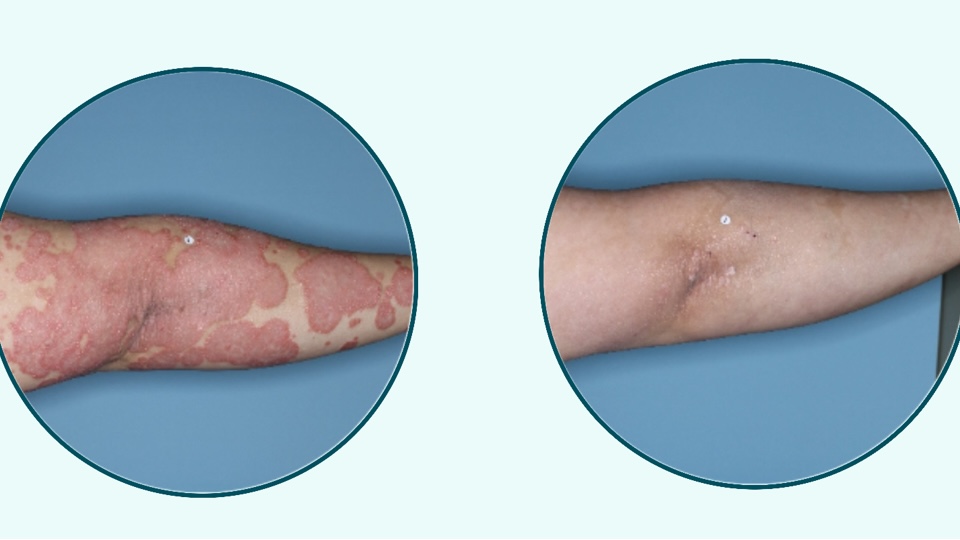
The case of the psoriasis antiinflammation antibody guselkumab is a story of deals and cooperation and selling and buying royalties to fuel other innovation paths. J&J is right in the middle of the game and has now full ownership while expanding the indications further.
Johnson & Johnson has announced positive topline results from its Phase 3b APEX study evaluating TREMFYA® (guselkumab) in adults with active psoriatic arthritis (PsA). The study met both its primary endpoint — reduction of signs and symptoms — and its major secondary endpoint — slowing of structural joint damage progression — at the 24-week mark, compared to placebo.
The APEX trial enrolled biologic-naïve patients who had not responded adequately to standard therapies such as csDMARDs, apremilast, or NSAIDs. TREMFYA®, a fully human, dual-acting monoclonal antibody, is the first and only approved PsA therapy that blocks IL-23 and binds to CD64, a receptor found on IL-23–producing cells such as activated monocytes and dendritic cells. IL-23 is a pro-inflammatory cytokine that plays a key role in immune-mediated diseases, including PsA.
The antibody was originally developed using the antibody technology platform of the German biotech company MorphoSys, based in Martinsried near Munich. MorphoSys was later acquired by Novartis for a single oncology asset it had previously obtained through its acquisition of Constellation Pharmaceuticals—a drug candidate that has yet to demonstrate success in gaining regulatory approval. To finance the Constellation deal, MorphoSys sold all milestone and royalty rights from its partnership with Johnson & Johnson, along with other asset-related agreements, to Royalty Pharma for US$300 million in 2022.
In late January, Royalty Pharma announced the sale of the remaining rights to these Tremfya-related royalties for approximately US$511 million to an undisclosed buyer. Now, Johnson & Johnson claims full marketing rights to this former MorphoSys product — strongly suggesting it may have been the undisclosed buyer in the transaction.
In APEX, patients treated with TREMFYA® demonstrated statistically significant improvement in joint symptoms and reduced radiographic progression of structural damage, as measured by the PsA-modified van der Heijde-Sharp (vdH-S) score, which includes joint space narrowing and erosion. These results reinforce the antibody’s ability to address both inflammation and long-term joint health.
No new safety signals were identified in the trial, and the findings were consistent with TREMFYA®’s well-established safety profile. The study includes a 24-week double-blind period, a 24-week active treatment phase, and a 12-week safety follow-up. Patients continuing into the long-term extension will receive an additional two years of active treatment, with final safety data collected thereafter.
Terence Rooney, Vice President and Rheumatology Disease Area Leader at Johnson & Johnson Innovative Medicine, emphasized the broader implications: “Psoriatic arthritis can lead to irreversible joint damage if not treated early. These new data highlight TREMFYA®’s unique ability to inhibit structural damage as the only IL-23 therapy to show such an effect—offering patients and providers a powerful tool to preserve joint function.”
TREMFYA® has previously shown efficacy in other immune-mediated diseases, including Crohn’s disease, where it outperformed Stelara in endoscopic endpoints during a Phase II/III trial. The company plans to present detailed APEX study results at upcoming medical congresses, further solidifying TREMFYA®’s role in advancing PsA treatment. With the success of APEX and a growing body of evidence, TREMFYA® continues to position itself as a cornerstone in the treatment of psoriatic arthritis and related inflammatory diseases.


 Freepik.com
Freepik.com adobe stock photos - Michael Derrer Fuchs
adobe stock photos - Michael Derrer Fuchs 