Halt of Polyphor trial shrinks company valuation
Roche had a nose for success when the pharma giant ended the CHF500m deal with Polyphor in November 2015. Now, the antibiotics maker stopped a Phase III trial of its lead murepavadin (Pol7080).
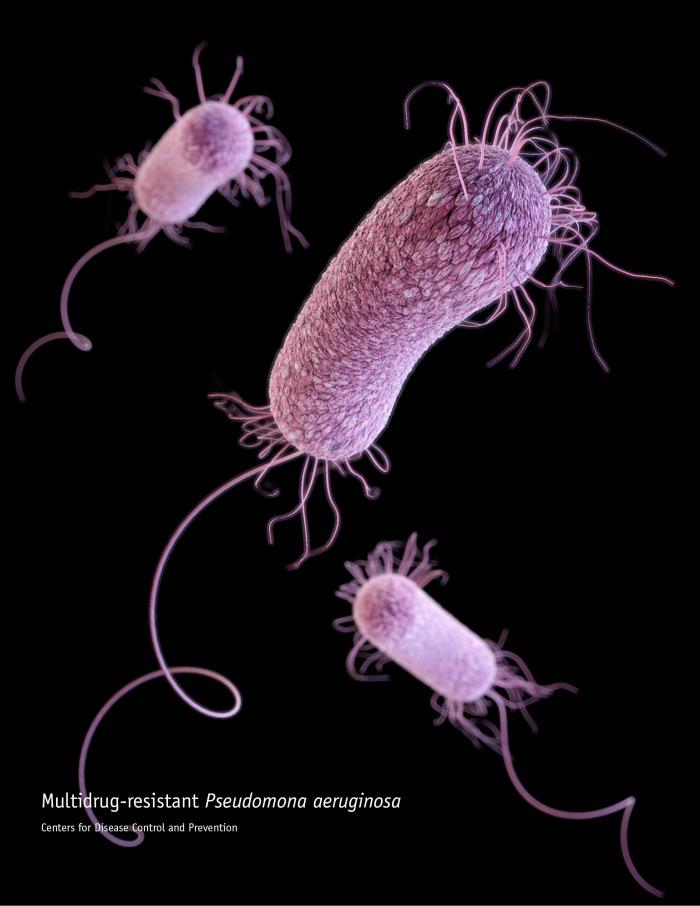
The announcement that Polyphor will halt enrolment for a pivotal Phase III study of its pneumonia antibiotic murepavadin (POL7080) due to unexpected high renal toxicity in the verum arm of the study shrank Polyphor’s value within hours by roughly 45%. After enrolment of 40 patients in the murepavadin arm, 32 (56.3%) of patients showed – partly advanced stage – akute kidney injury (AKI) vs 25% of patients in the placebo arm while mortality was identical in both treatment arms.
Murepavadin was a white hope against antibiotic-resistant Gram-negative bugs. Targeting the OstA protein of the lipopolysaccharide layer of the outer Pseudomonas membrane, the peptide drug promised to be a rare weapon in the global fight of antimicrobial resistance.
.


 Sitryx Therapeutics
Sitryx Therapeutics
