FDA approves cell therapy for solid tumours
The US Food & Drug Administration has granted accelerated approval to Adaptimmune's Tecelra as the world's first therapy with genetically engineered T cells to fight a solid tumour.
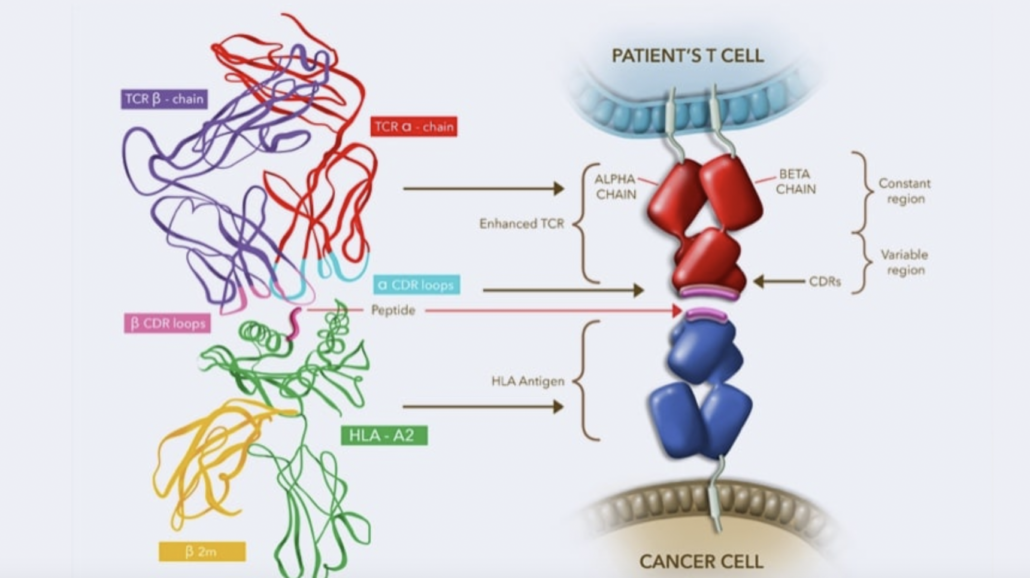
Until now, the success of CAR-T or TCR-T cell therapies has been limited to blood cancers. Despite numerous clinical trials, their benefit in the treatment of solid tumours, which account for more than 90% of all cancers, remained unclear. This is now history with the accelerated approval of the TCR-T therapy afamitresgene autoleucel (ADP-A2M4, Tecelra) from UK-based Adaptimmune Ltd for the treatment of HLA-A*02-positive, metastatic or unresectable synovial sarcoma, which is directed against the MAGE-A4 antigen frequently expressed on tumours.
In February, the FDA granted accelerated approval to the cell therapy lifileucel from Iovance Biotherapeutics Inc as a second-line treatment for inoperable malignant melanoma. However, the tumour-infiltrating lymphocytes isolated from tumour cells floating in the blood and then multiplied are not genetically modified like Adaptimmune and are therefore – contrary to what Iovance claims – in no way similar to CAR-T or TCR-T cell therapies, except for the fact that they are autologous.
However, following pre-approval of the autologous therapy, which is based solely on clinical evidence of a 39% response rate and increased tumour infiltration, Adaptimmune must provide efficacy data in order to receive full approval. Crucial for the accelerated approval: it is the first new therapy for synovial sarcoma in ten years.
In April this year, Roche’s US subsidiary terminated a collaboration with Genentech Inc in 2021 to develop five MAGE- A4-based allogeneic TCR-T therapies based on induced pluripotent stem cells (hiIPSCs).
The list price for the one-off autologous therapy is USD 727,000. In the USA, there are around 400 patients per year who are eligible for treatment.


 Araris Biotech AG
Araris Biotech AG Roche
Roche ABA Austria
ABA Austria