Mysthera Therapeutics AG has launched in Basel with $3.5m in seed funding from founding investor Forty51 Ventures. The capital will be used to advance its preclinical portfolio in a variety of autoimmune indications. The focus is on pan-PIM kinase inhibitors that uniquely modulate multilineage immune cell functions.
ADVERTISEMENT
Women’s health specialist Mithra Pharmaceuticals SA has entered into a definitive agreement to raise €20m in gross proceeds via a private placement of 10 million new ordinary shares with Armistice Capital.
Researchers at the Universities of Bonn (Germany) and Utrecht (The Netherlands and from NovoBiotic Pharmaceuticals (Cambridge, US) have discovered a new class of antibiotics. They isolated clovibactin from the thought-to-be uncultured soil bacterium Eleftheria terrae, subspecies Carolin. It efficiently kills drug-resistant Gram-positive bacterial pathogens without detectable resistance.
Using biochemical assays, solid-state nuclear magnetic resonance, and atomic force microscopy, the team dissect its mode of action. Clovibactin blocks cell wall synthesis by targeting pyrophosphate of multiple essential peptidoglycan precursors (C55PP, lipid II, and lipid IIIWTA). Clovibactin uses an unusual hydrophobic interface to tightly wrap around pyrophosphate but bypasses the variable structural elements of precursors, accounting for the lack of resistance. Selective and efficient target binding is achieved by the sequestration of precursors into supramolecular fibrils that only form on bacterial membranes that contain lipid-anchored pyrophosphate groups. This potent antibiotic holds the promise of enabling the design of improved therapeutics that kill bacterial pathogens without resistance development.
The discovery of clovibactin was made possible by a new technology called the iCHip apparatus. This makes it possible to grow bacteria in the laboratory that were previously considered unculturable.. The mechanism of action of the new antibiotic was elucidated by researchers led by Tanja Schneider of the Institute of Pharmaceutical Microbiology at the University Hospital Bonn. They showed that Clovibactin blocks the synthesis of the bacterial cell wall by binding specifically and with high specificity to pyrophosphate groups on cell wall building blocks (peptidoglycan precursors).
After docking with the target structures, Clovibactin forms supramolecular fiber-like structures that tghtly enclose the pyrophosphate groups – like a cage. Hence the name of the new antibiotic, which is derived from the Greek word "Klouvi" (cage). Bacteria that encounter Clovibactin are also stimulated to release autolysins, which uncontrollably dissolve their own cell wall.
Clovibactin acts primarily on gram-positive bacteria. These include methicillin-resistant Staphylococcus aureus (MRSA), as well as the pathogens that cause tuberculosis. "We are very confident that the bacteria will not develop resistance to clovibactin as quickly," Schneider says. He adds that the combination of the different mechanisms of action results in "exceptional resistance to resistance."
Moreover, the pyrophosphate groups to which Clovibactin docks are immutable. This leaves the bacterium with few options to escape the antibiotic through mutation. Next, the research team plans to use its findings to date to further increase the effectiveness of Clovibactin. However, Schneider says there is still a long way to go before the new antibiotic reaches the market.
Researchers at Northeastern University have previously used the iChip to find another antibiotic called teixobactin.
The UK is making €15m investment in its biopharmaceutical manufacturing industry via its national innovation agency Innovate UK. It is part of the country’s larger push to support the domestic Life Sciences.
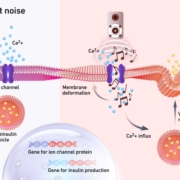
ETH Zurich researchers have developed a gene switch that triggers insulin release in designer cells by playing music.
British CDMO Oxford Biomedica plc announces the expansion of its License and Supply Agreement with cell therapy specialist Cabaletta Bio, Inc. The new agreements adds CD19 as a new target – it originally covered Cabaletta Bio’s lead product, DSG3-CAART.
Oil-eating bacteria form dendritic biofilms that reshape oil droplets to speed up the rate of consumption
French vaccine maker Valneva SE will double the amount of a US$100m debt financing granted by Deerfield Management Company and OrbiMed.
Swiss Spexis AG has announced a US$2.5m capital commitment to fund a two-part pivotal Phase III study to get market approval for its inhaled cystic fibrosis antibiotic colistimethate sodium.
With a US$1bn acquisition of Canadian Inversago Pharma, Novo Nordisk A/S fiights to maintain its dominant position in the booming weight loss drug market.