Following the birth of genome-edited twins in China, the German Ethics Council has provided a complete ethical review about human germline intervention.
ADVERTISEMENT
Epigenetic changes in immune cells may provide the trigger of multiple sclerosis. Researchers have now identified MS-specific epigenetic biomarkers that change upon drug treatment.
The round was led by BioGeneration Ventures and Wellington Partners, with Fund+, Perceptive Advisors and existing investors Capricorn Venture Partners, MINTS, PMV, QBIC and V-Bio Ventures contributing.
US scientists have found that the complex interactions between fat tissue and the immune system are mediated by interleukin 33 (IL-33).
The European Lead Factory (ELF) – a drug discovery hub for validation new targets from academia and biotech SMEs – has again received project funding from the Innovative Medicine’s Initiative.
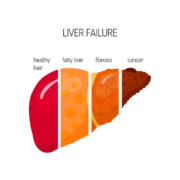
Liver specialist Alentis Therapeutics AG has raised CHF12.5m in a Series A round co-led by BioMedPartners and BB Pureos Bioventures.
In its first meeting in Amsterdam, the CHMP of the European Medicines Agency recommended six new drugs, two orphan meds, and one biosimilar for EU market authorisation.
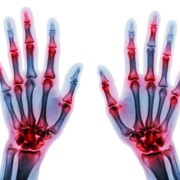
Researchers at AstraZeneca, Viela Bio, Gilead Sciences, Antidote Therapeutics Inc., and at SoseiHeptares report on a new small molecule compound to treat RA.