Researchers at Novavax and from the University of Oxford reported an efficacy of 77% of their malaria vaccine, R21/Matrix-M from a Phase II trials in Burkina Faso.
ADVERTISEMENT
GSK’s and Vir Therapeutics’ COVID-19 antibody treatment VIR-7831 has entered Phase III testing within the UK’s COVID-19 drug testing platform, AGILE
After a positive re-evaluation of J&J’s COVID-19 vaccine by the European Medicines Agency (EMA), J&J has re-started to ship 55 million doses to the EU.
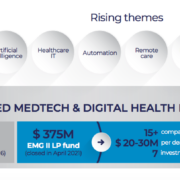
Endeavour Vision has announced the closing of its Heathtech fund Endeavour Medtech Growth II (EMG II) LP at US$375 million in capital commitments.
The Swiss biotech industry experienced a record investment in the pandemic year 2020, according to the new Swiss Biotech Report.
A new study on the persistence of neutralising antibodies to SARS-CoV-2 in the blood suggests that humoral immunity will drop 5 months after vaccination.
Swiss BioVersys has received €20mfrom the European Investment Bank to push the development of new drugs against life-threatening nosocomial infections.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.
C4X Discovery Holdings plc has licenced its oral C4XD oral IL-17A inhibitor programme to Sanofi.