Why the US AMR accelerator aims to expand its reach in Europe to support antibacterial R&D, and why it is important to bundle global resources, explains Kevin Outterson, Executive Director of CARB-X.
ADVERTISEMENT
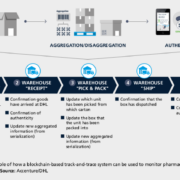
Its only been nine years since the first blockchain technology applications in finance were introduced. Big players are now working to use distributed computing to protect pharma logistics from counterfeit drugs. Unlike current Internet-based solutions, the decentralised blockchain network is immune to manipulation or hacks, as transactions can be verified, recorded, and coordinated autonomously without third-party involvement.
After a quick start out the gates in 2012, the European Commission proceeded to carry out a lengthy review of its bioeconomy strategy – slamming the brakes on investor interest in EU biotechs developing solutions for the move to a low-carbon, zero-waste economy. Following several reports that suggested the bloc will miss internationally agreed sustainability targets, the EC now wants to speed up the switch to biobased production. Industry stakeholders presented a huge range of promising technologies at the EFIB, but are still awaiting the go-ahead.
Take the right dataset, add an ingenious algorithm or two – and voilá! Your billion-euro blockbuster molecule is ready! AI-driven drug development is exciting investor interest, while Big Pharma is also asking what forms of artifical intelligence can be slotted in at what stages in the process. AI-powered drug repurposing has seen some candidates enter clinical stage testing, but it’s still unclear whether the technology is already powerful enough for de novo molecule discovery.
It’s the most commonly cultivated, trafficked and abused illicit drug worldwide, and there’s still a lot of social stigma attached to the recreational use of pot. But it’s slowly dawning on regulators in countries from Canada to the US to South Africa that by ignoring the clear medical benefits of marijuana, they may have thrown the baby out with the bathwater. Countries across Europe are now also opening their minds, laws and wallets to cannabis.
According to a brand-new EU-wide survey, European plant researchers are concerned about the European Court of Justices (CJEU) interpretation that targeted mutation methods aimed at improving crop yield and resistance to climate change yield GMOs. In an open letter, they call for political action and a modernisation of the EU Directive 2001/18/EC because a lack in tech-transfer would put Europe at the bottom of the pile in the upcoming bioeconomy.
Datwyler Sealing Solutions is a leading industrial supplier and a key player in the global health care world. Our unique product range includes the most advanced elastomer formulations, coatings, aluminum seals, and processing technologies. These are based on a broad spectrum of experience in the healthcare industry. To ensure the highest quality in our broad product portfolio, we have implemented the most advanced manufacturing standard.
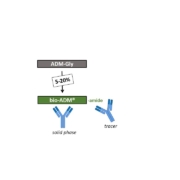
Bio-ADM, the very first blood biomarker capable to diagnose when blood vessels become leaky (endothelial dysfunction), is set to make a rapid carrier at intensive care units (ICUs) and emergency departments (EDs) of hospitals. Data from pilot clinical routine testing presented at the 2nd Scientific Symposium on Endothelial Disruption (18. December 2018) in Berlin impressively confirmed results from previous tests on 30,000 patients with acute heart failure (AHF) and severe sepsis indicating that bio-ADM testing improves medical decision making to prevent mortality, organ damage and rehospitalisation. An automated point-of-care (POC) test measuring bio-ADM both as a biomarker and as a companion diagnostic to a therapeutic antibody (adrecizumab) that repairs endothelial dysfunction, will be launched by H1/2019 by Sphingotec GmbH. However, bio-ADM is just the first representative of a full pipeline of diagnostic blood proteins reflecting disturbed signalling pathways underlying disorders of unmet medical need.
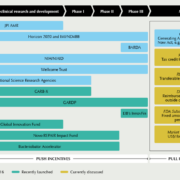
In autumn, stakeholders across the globe pledged to take more action in the fight against antimicrobial resistance (AMR). Whereas from the push side new public and private initiatives appeared on the screen, discussion about which pull mechanism should be started first is in full swing. Meanwhile, small and medium-sized companies combine forces aiming to propel preclinical and early clinical R&D.
Diagnostic results must be readily available to diagnose critically ill patients in emergency settings and at ICUs. So far, diagnostic blood tests, which demonstrably could help emergency meds recognise residual congestion in heart failure, septic shock, or acute kidney injury before patients reach critical condition, have not been available in the automated format required at ICUs. After Sphingotec acquired Nexus IB-10 POC testing platform in May, investors wait in line.