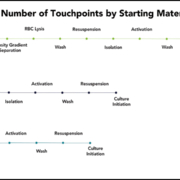
Isolating cells from whole blood or LRS cones carries the risk of red blood cell (RBC) contamination, which can have deleterious effects on downstream cell cultures.
ADVERTISEMENT
The regulatory situation regarding the trade of medicinal cannabis currently varies enormously from country to country within the European Union. Unfortunately, there is still no full regulatory harmonization on a European level.
Effective bioprocess development starts with the targeted and time-saving generation of high-productivity strains already considering economic target values and regulatory requirements. The UNLOCK PICHIA technology platform comprises a large number of versatile expression tools for the generation and identification of effective industrial protein production strains without compromising development timelines.
The future of AAV vector-based therapies holds great promise if manufacturers can cope with the demand. While in-house manufacturing may be an option for some companies, partnering with a CDMO can be the best choice for others. Teaming up with a One-Stop-Shop CDMO can make it easier to achieve the project goals. There are many reasons for this.
The German Patent System has been a very patentee-friendly system, in particular for smaller patentees. Enforcement of patents has been relatively easy, affordable and fast.
Study start-up is a chaotic time. There are lots of moving parts. Lots of decisions to make and processes to establish. Lots of room for error. Mistakes can quickly derail a trial – and delays are endemic. That all represents a potential waste of time and resources – just at a moment when speed is of the essence. In many cases, the problem comes down to spreadsheets.
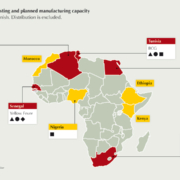
Africa produces less than 1% of the human vaccines it uses. To effectively respond in time of outbreaks and pandemics, Africa must expand its vaccine development and production capabilities to cater for its growing population. Successfully establishing such an industry at scale is a multifaceted endeavour to create an enabling environment. This article highlights key factors and considerations for establishing local vaccine manufacturing.
IP – In a recent decision (Royalty vs. DPMA; C-650/17), the European Court of Justice (ECJ) has shed some light on the conditions under which a supplementary protection certificate (SPC) may be granted. It has thus ended discussions that had arisen following earlier somewhat elusive ECJ-decisions. Whether this will be the last word to be heard on the topic remains to be seen.
15min to detect bacterial infections – that’s what British diagnostics company FluoretiQ Ltd is offering in the fight against antimicrobial resistance. A short interview with CEO Neciah Dorh.
Interview with Ian Harrow from the Pistoia Alliance, about how to improve life sciences data management with help of a new digital toolkit that makes data Findable, Accessible, Interoperable, and Reusable – in brief: FAIR.