A novel BPM system
The biotech and pharma branch is growing steadily. Many companies have full order books for the next few years and expect growth. This is the basis for new investments in assets and infrastructure. These growing structures often expose vulnerabilities and challenges for the business. Therefore, companies need to increase the stability and efficiency of their processes.
Richter-Helm is one of the companies currently expanding to serve the needs of the biotech and pharma market. There will be a significant increase in manufacturing capacity at the company´s production site in Bovenau. The new building will be one of the most modern facilities in the world and perfectly meets the company’s high standards in GMP-compliant CDMO business. It will be ready for operation this year. The current challenge is to transfer the well working processes to the new facility. Thereby, Richter-Helm is continuously improving and standardising the processes of the entire company.
Richter-Helm will be able to differentiate itself from the competition in the CDMO business and respond more flexibly to the needs of its customers.
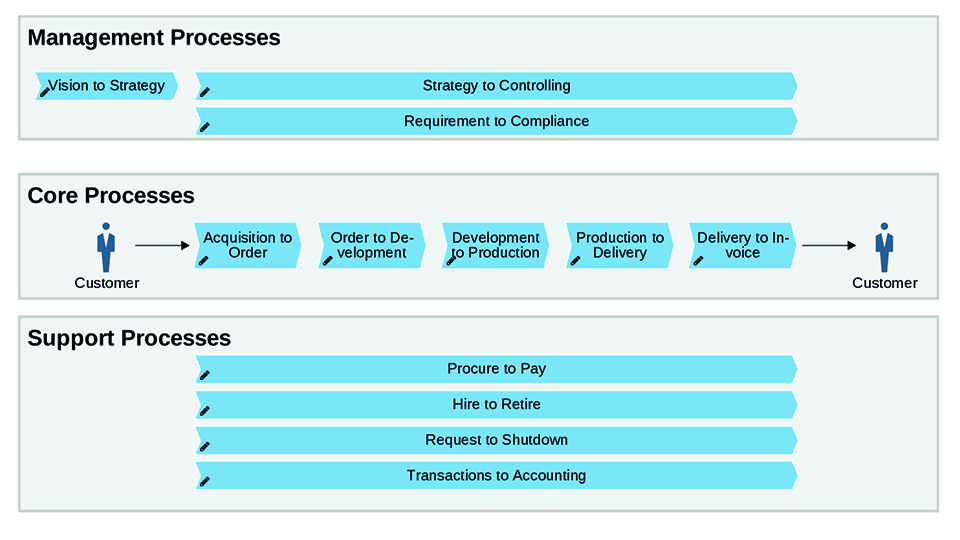
Business Process Management (BPM) is the structured approach to achieving this goal by making activities, responsibilities and interfaces within an organisation transparent. Implementation begins with the documentation of business processes. This is the basis for later analysis and optimisation. It is recommended to start with a top-down definition of the most important processes. The result is the process landscape which represents the main processes. The next step is the detailing. Therefore, the worldwide standard BPMN 2.0 (Business Process Modelling Notation) which is used by most of the companies can be used. The introduction of a BPM software is recommended to simplify the modelling.
During the modelling, companies with several locations often face the problem that their processes work differently. Different executions of the same process can lead to variations in the quality of the products delivered to the customer. This may affect the reputation of the company and the customer satisfaction. So, the first step should be the standardisation to increase the efficiency of the process and the quality of the output. In this way, sites can learn from each other and adopt best practice. At first glance, this may seem like a simple quick win, but it requires very good change management to get everyone on board. It is therefore important to involve representatives from affected sites as early as possible. Otherwise, the standardisation will only be valid on paper, but won’t be implemented. It is also important not to force standardisation on every process. The implementation of BPM is an ongoing process to continuously improve the maturity of the organisation. The North Star is the achievement of the level “Continuous Improvement”. This level is never finished, because process improvement doesn’t stop. It is always assumed that the current process is the worst process and must be improved.
This article was orginally published in European Biotechnology Magazine Spring 2024.


 Lonza Group
Lonza Group Vetter Pharma
Vetter Pharma Rentschler Biopharma SE
Rentschler Biopharma SE