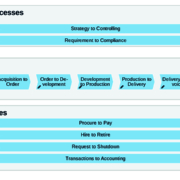
The biotech and pharma branch is growing steadily. Many companies have full order books for the next few years and expect growth. This is the basis for new investments in assets and infrastructure. These growing structures often expose vulnerabilities and challenges for the business. Therefore, companies need to increase the stability and efficiency of their processes.
ADVERTISEMENT
Tag Archive for: Standardisation
Precision medicine tailors therapeutics to individual patients’ diseases, and is seen as the future of clinical practice. With significant time and financial investments being made in developing therapeutics, the ultimate success of patient-data driven precision therapies hinges on three main factors: the quality of the tissue samples, the associated molecular data, and the analysis used in their development.
The Sweden Biobank Act governs the collection, storage, and usage of identifiable human material samples for specific purposes.