
Alnylam in US$2.8bn biobucks deal with Roche
siRNA specialist Alnylam Pharmaceuticals Roche has received US$310m upfront from Swiss Roche AG for the ex-US commercialisation rights of zilebesiran, an new antihypertensive. T
The deal, which could fetch Alnylam Pharmaceuticals Inc up to US$2.8bn if all milestones are met and marketing approval is granted, clearly marks Big Pharma’s return to cardiology research, which was written off decades ago. Latest Phase II data presented by the US siRNA specialist demonstrate a clear antihypertensive effect of zilebesiran with an acceptable side effect profile. The drug dose-dependently reduces serum angiotensinogen levels.
Under the ageement, the Swiss pharmaceutical company will pay the major part of costs for a large clinical trial to test whether zilebesiran can lower the risk of dangerous cardiovascular events like heart attacks and strokes. Headline results of two ongoing Phase II studies are expected in mid-2023.
After years of prioritising oncology and immunology on cost of cardiology, Big pharma is renewing its investments in cardiology due to better stratification, thus smaller and real-world data supported clinical trails that significantly reduce costs. While Novartis bought Leqvio developer The Medicines Company, BMS took over heart failure specialist Myokardia, which got market authorisation for Camzyos. Other companies such as Amgen or Merck & Co are recently adapting its pipelines.


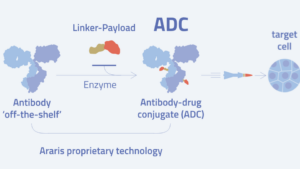 Araris Biotech AG
Araris Biotech AG Roche
Roche BioNTech SE
BioNTech SE