Servier acquires Kaerus Bioscience’s FXS candidate for US$450m
In a US$450m deal with UK-based Kaerus Bioscience Ltd, Servier SA has acquired the development and commercialisation rights to KER-0193, a Phase II-ready drug candidate for the treatment of rare Fragile X syndrome, the most common monogenic cause of autism.
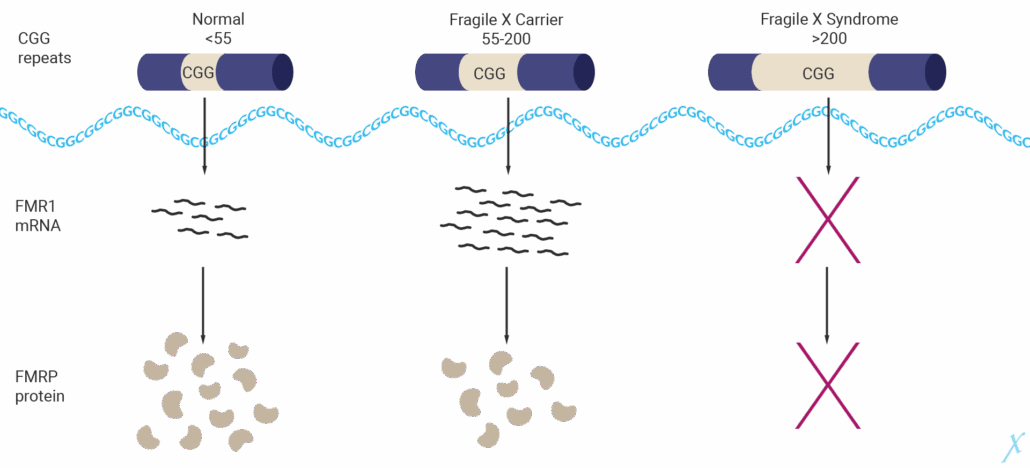
Fragile X syndrome is a monogenic model for autism in which the loss of the RNA-binding protein FMRP as a result of silencing of the mutant transcription regulator gene FMR1 leads to dysregulated synaptic protein synthesis, impaired neural plasticity and autism-like behavioural phenotypes.
With the US$450 million acquisition of KER-0193 from Medicxi subsidiary Kaerus Bioscience, French oncology play Servier SA is securing a foothold in a market that is much larger than the US$318.5 million that Global Data currently attributes to the rare fragile X syndrome (FXS). This is because 20% of autism patients have overlapping symptoms with FXS.
Kaerus Bioscience successfully completed Phase I safety trials with KER-0193 in March 2025, demonstrating good tolerability and excellent pharmacokinetics. In preclinical trials, the BK channel protein inhibitor showed an improvement in syndrome-related behavioural, sensory and cognitive deficits. KER-0193 has FDA Orphan Drug and Rare Paediatric Drug status for the treatment of FXS. Servier plans to start a Phase II trial next year.
KER-0193 is competing to become the first globally approved FXS therapy with the unpartnered US start-up Sphinogenix Inc, whose candidate SPG-601, which binds the same target as KER0193, has not only completed a small Phase II trial (n=10) but also received FDA Fast Track status in December. However, the study only recorded EEG changes and no syndrome modification.
In addition, Shionogi Inc/Tetra Therapeutics achieved significant improvements in speech and social interactions in completed Phase II studies with its selective PDE4D inhibitor zatomilast (BPN14770). An mRNA gene therapy aiming at restoring FMR1 gene expression is still in the discovery stage at QurAlis Corporation.



 VFA
VFA gov.uk
gov.uk