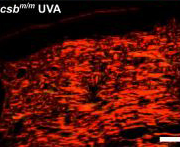
An FDA-approved cancer drug stops light sensitivity of the skin in Cockayne syndrome (CS), an ultra-rare hereditary disease characterised by premature aging, German researchers report.
ADVERTISEMENT
Germany’s government announced that it will create an agency for breakthrough ("jump") innovation to foster technology transfer of risky research projects with high market potential into products.
Roche today announced that Roland Diggelmann, CEO Diagnostics Division and member of the Corporate Executive Committee, will be leaving Roche to pursue his career outside of the company effective 30 September 2018. Until a successor is named, Michael Heuer, currently Region Head Europe, Middle East, Africa, and Latin America for Roche Diagnostics will assume the ad interim leadership of Roche’s Diagnostics Division and become a member of the Corporate Executive Committee.
Congenica today announced the appointment of Mrs Wendy Britten as its new Chief Financial Officer (CFO), effective 1st September, 2018. Mrs Britten will have overall control and responsibility for all financial aspects of the business and will play a key role in business development and fundraising activities.
Roche’s US arm Genentech has licenced Affimed NV’s ROCK platform to add multivalent antibodies, which could activate the innate immune system, to its cancer pipeline.
The British Heart Foundation (BHF) will tender £30m (€33.2m) to tackle the suffering and devastation caused by heart and circulatory diseases.
About 25% of the people who suffer from haemophilia A have no therapeutic options, because their immune system forms antibodies against clotting products that can stop bleeding. Researchers and companies in the growing US$15.8bn haemophilia market are feverishly exploring new ways to identify patients at risk and offer them alternative treatments. A series of mergers and acquisitions have changed the playing field for established markets and players.
Orphan drug leader Shire plc (Dublin), has received the FDA’s approval for its antibody lanadelumab-flyo, which prevents hereditary angioedema (HAE) attacks.
Blocking the extracellular matrix (ECM) enzyme leukotriene A4 hydrolase (LTA4H) dampens inflammation but promotes changes in the airways of mice with allergic asthma due to accumulation of the ECM degradation product PGP.